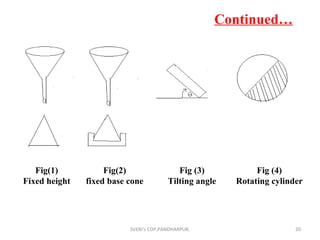
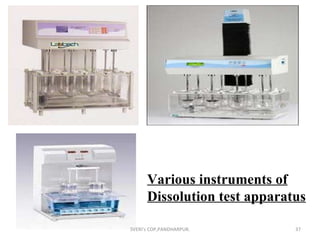
The document discusses characterization techniques for pharmaceutical granules and compacts. It describes methods for analyzing granule particle size, shape, density, moisture content, flow properties, and friability. It also covers techniques for evaluating tablet properties like weight variation, disintegration, dissolution, hardness and thickness. The purpose is to ensure granules and tablets meet specifications for content uniformity, drug release and stability.