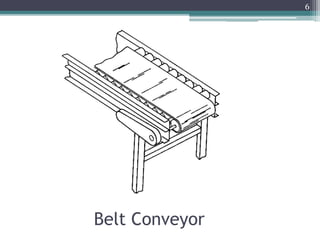
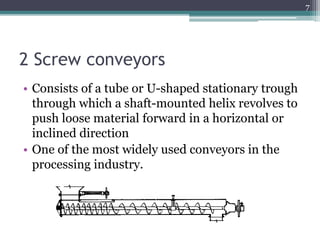
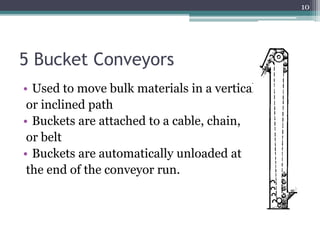
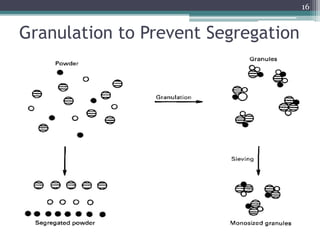
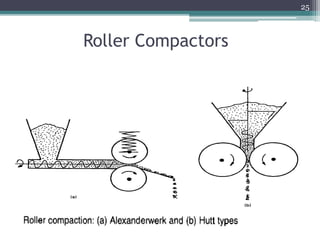
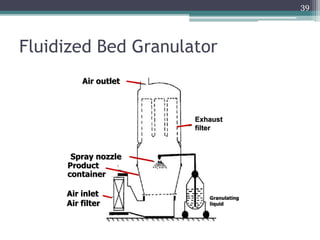
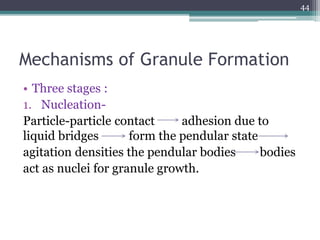
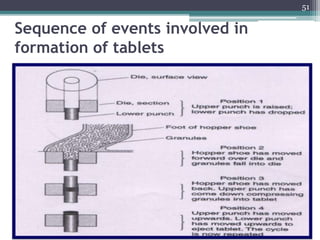
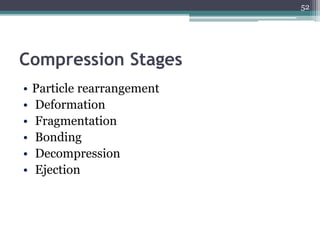
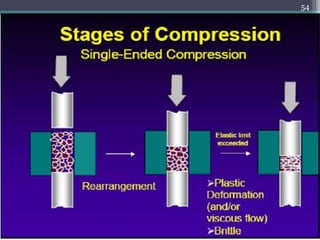
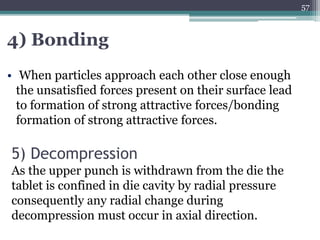
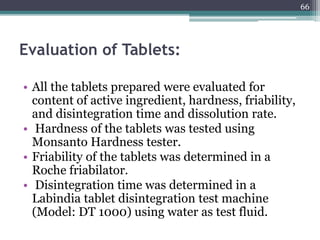
This document summarizes a case study comparing direct compression and wet granulation methods for formulating stavudine tablets. Materials used include stavudine, acacia, PVP K30, lactose, crospovidone, talc, and magnesium stearate. Tablets were prepared by either directly compressing blended powders or wet granulating powders with water and drying the granules before compression. The resulting tablets from both methods were then evaluated for drug content, hardness, friability, and disintegration time.