Embed presentation










































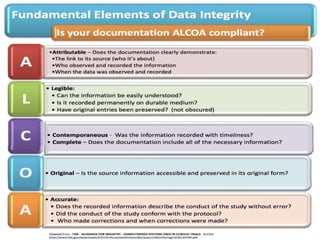
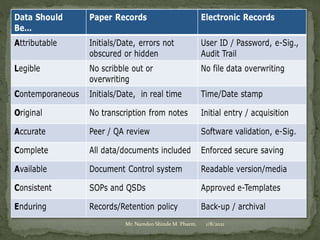


Mr. Namdeo Shinde gave a presentation on good documentation practices in the pharmaceutical industry. He discussed how a lack of documentation led to deaths in a 1972 incident in the UK. Proper documentation is necessary for GMP compliance and provides an audit trail for regulators. Documentation must be systematically prepared, verified, stored and reviewed. The most important documents include standard operating procedures, batch manufacturing records, test methods and specifications. All activities must be documented according to GMP guidelines to ensure quality and prevent errors.












































