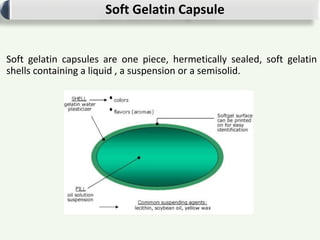
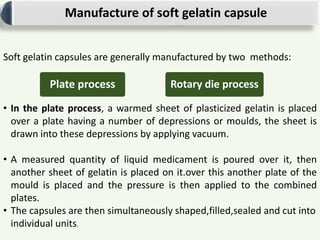
Soft gelatin capsules are solid dosage forms where the drug is enclosed in a soft soluble gelatin shell, usually formed from gelatin. There are two main types of capsules: hard gelatin capsules and soft gelatin capsules. Soft gelatin capsules are one-piece shells containing liquids, suspensions, or semisolids. They are manufactured using either a plate process or rotary die process, which simultaneously fills, seals, and cuts the capsules. Quality is ensured through testing of ingredients, in-process testing, and finished product testing.