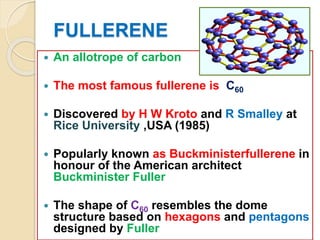
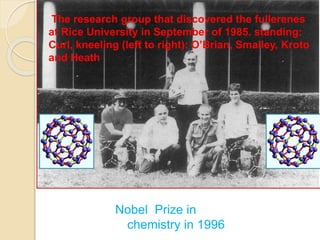
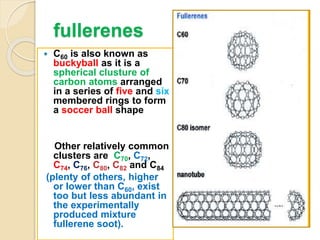
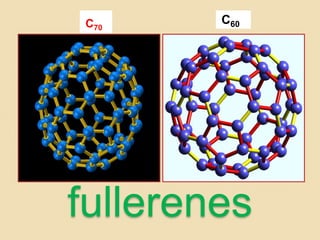
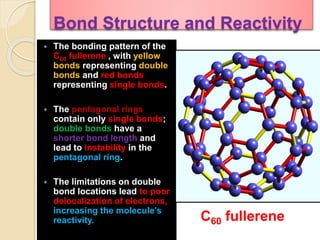
This document discusses fullerenes, an allotrope of carbon. It describes how the most common fullerene, C60, was discovered in 1985. C60 resembles a soccer ball shape made of carbon atoms arranged in hexagons and pentagons. The document outlines the structure and properties of fullerenes like C60, as well as methods for synthesizing and purifying fullerenes. Potential applications mentioned include use as lubricants, in electronics, and for inhibiting HIV.