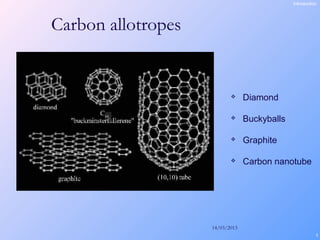
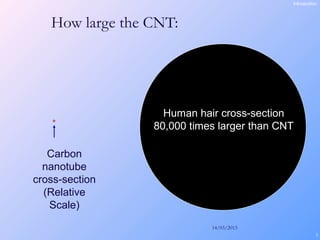
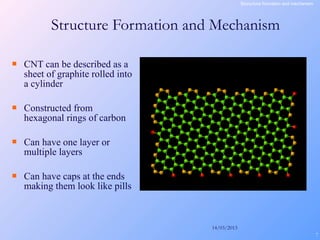
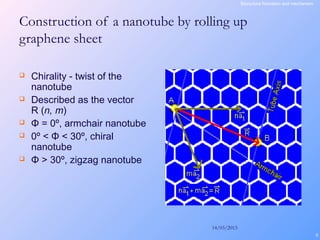
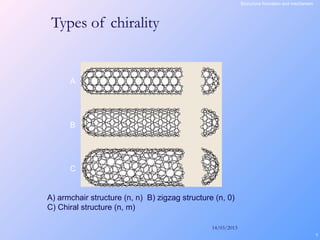
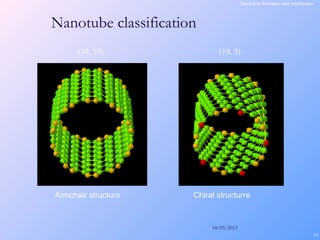
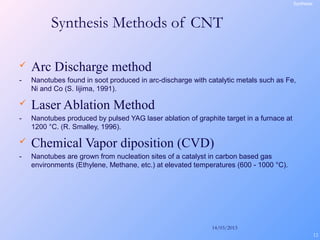
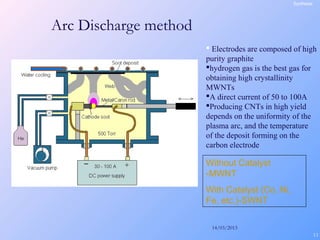
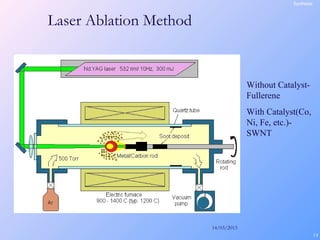
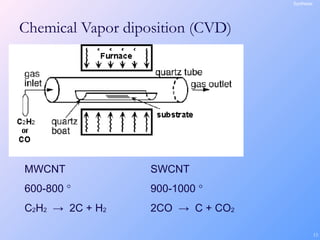
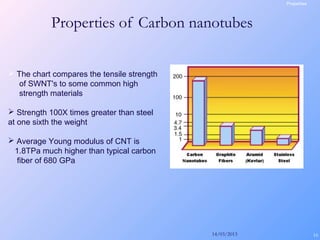
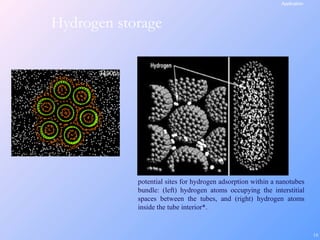
This document is a report on carbon nanotubes written by Ajay Pandey for a class at the University of Mumbai. It begins with an introduction to carbon nanotubes, describing their tubular structure and unique properties. The report then covers carbon nanotube structure formation mechanisms, various synthesis methods including arc discharge and chemical vapor deposition, and properties such as strength and conductivity. Applications discussed include energy storage, electronics, sensors and composite materials. The report concludes that carbon nanotubes have great potential in nanotechnology due to their robust structure and properties, but that further study is still needed on issues like functionalization and biocompatibility.