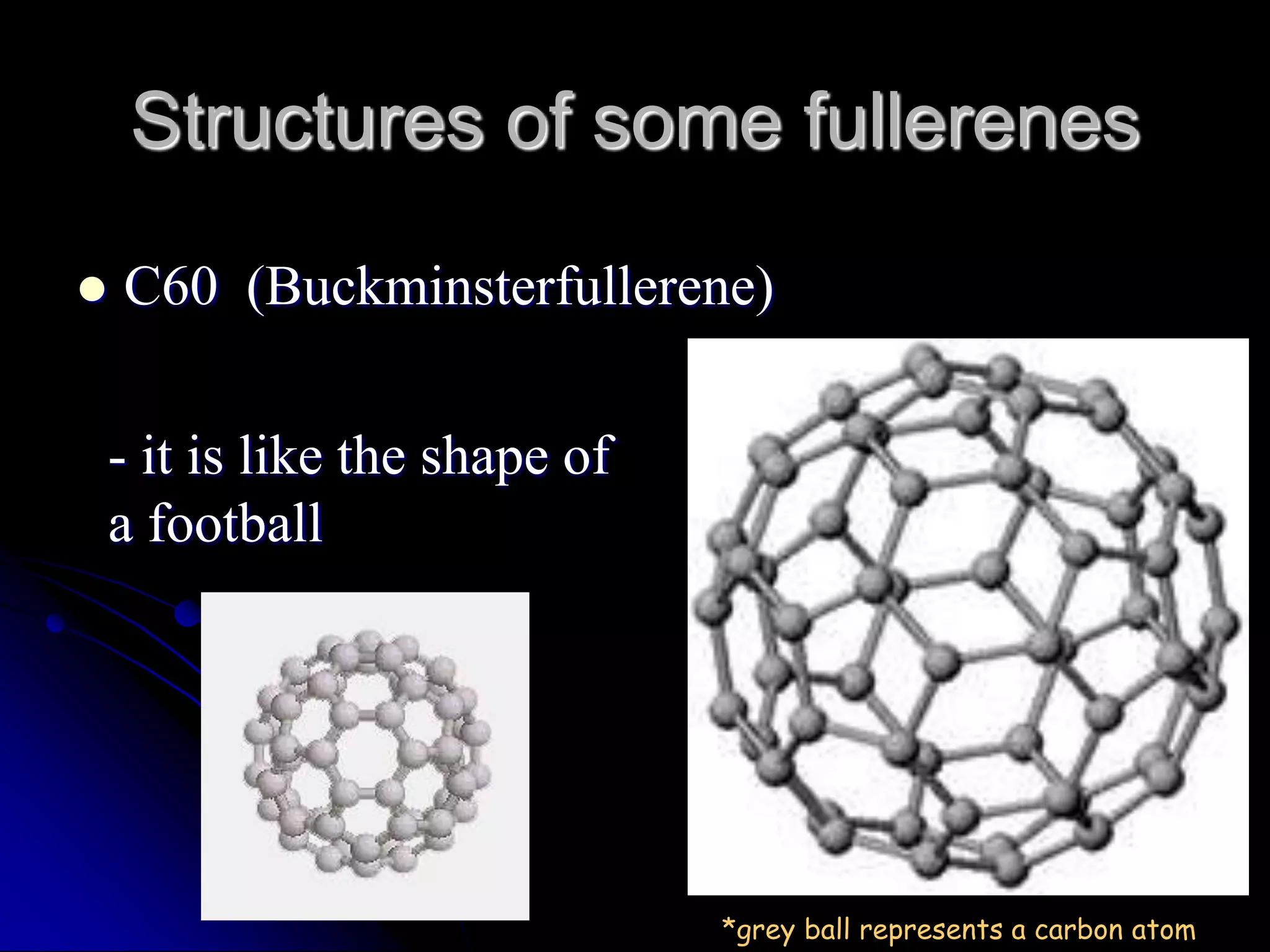
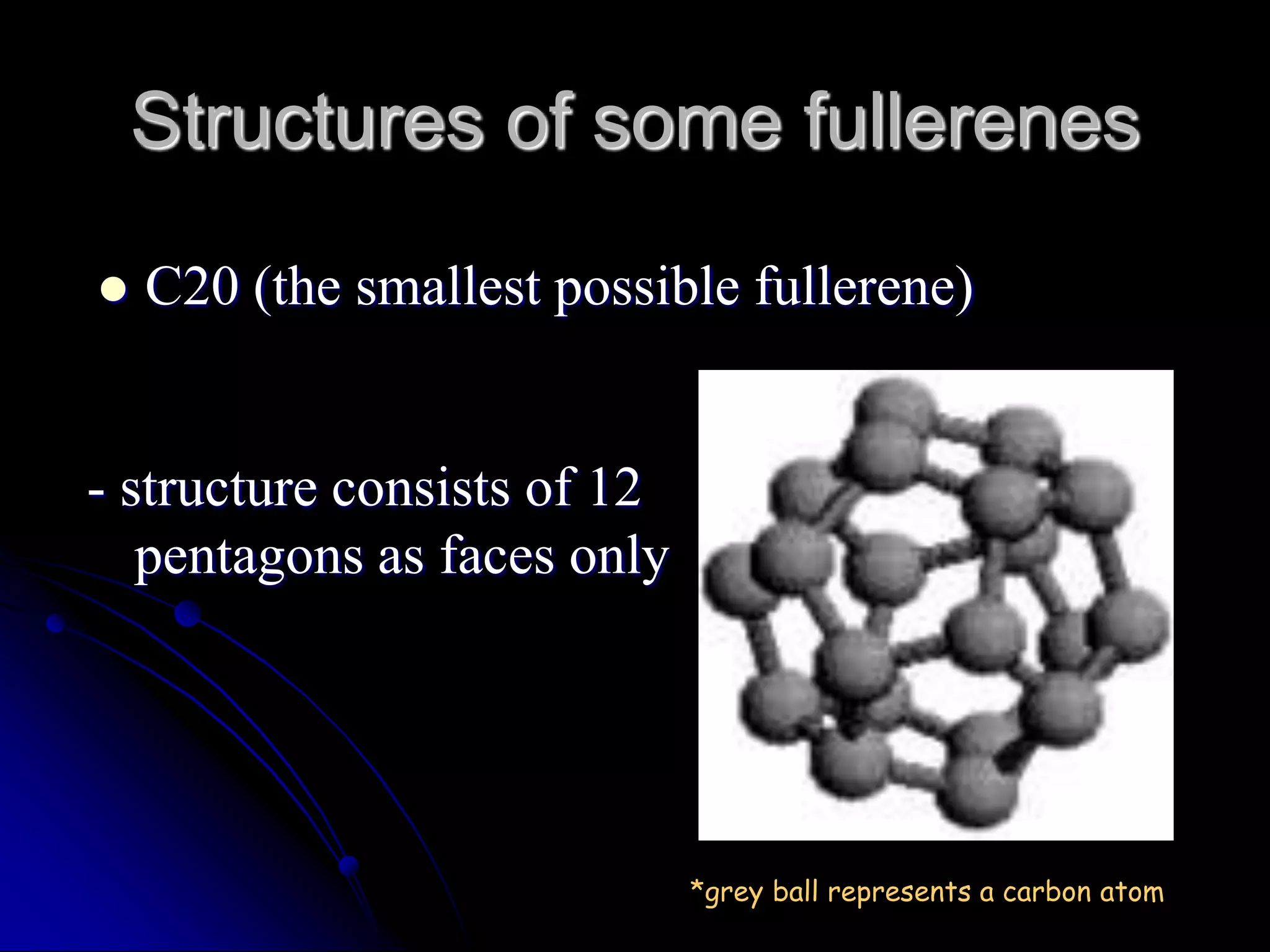
Fullerenes are carbon allotropes composed entirely of carbon in hollow spherical, ellipsoid, or tubular structures. In 1985, Kroto, Curl, and Smalley discovered buckminsterfullerene (C60) through molecular beam experiments that observed discrete carbon molecule peaks, including at 60 atoms. C60 is named after geodesic dome architect Buckminster Fuller due to its similar structure. Fullerenes have applications as polymer additives, in carbon nanotubes used for strong, conductive materials like tennis rackets and aircraft composites, and in molecular pumps and nanowires for electrical motors and light bulbs.