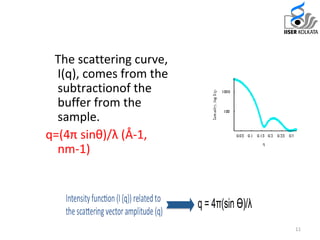
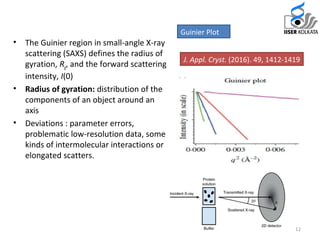
Small Angle X-ray Scattering (SAXS) is a technique that provides structural information about macromolecules within a size range of 5 to 25 nm and can analyze larger dimensions with ultra-SAXS. The document details the history, applications, and advantages of SAXS compared to traditional X-ray diffraction, highlighting its suitability for flexible proteins that don’t crystallize easily. SAXS is shown to be a complementary method, requiring minimal sample volume and providing rapid data collection.