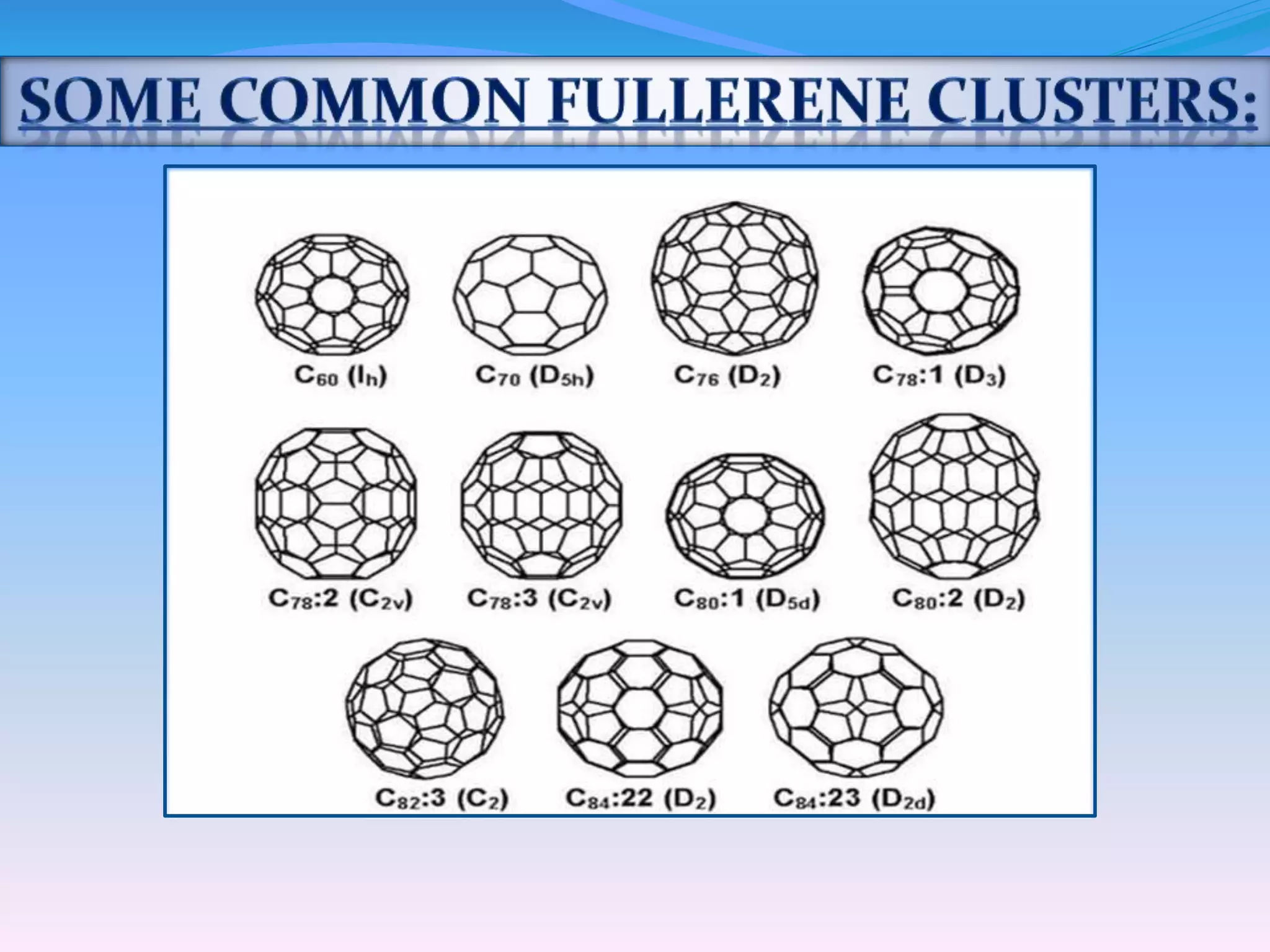
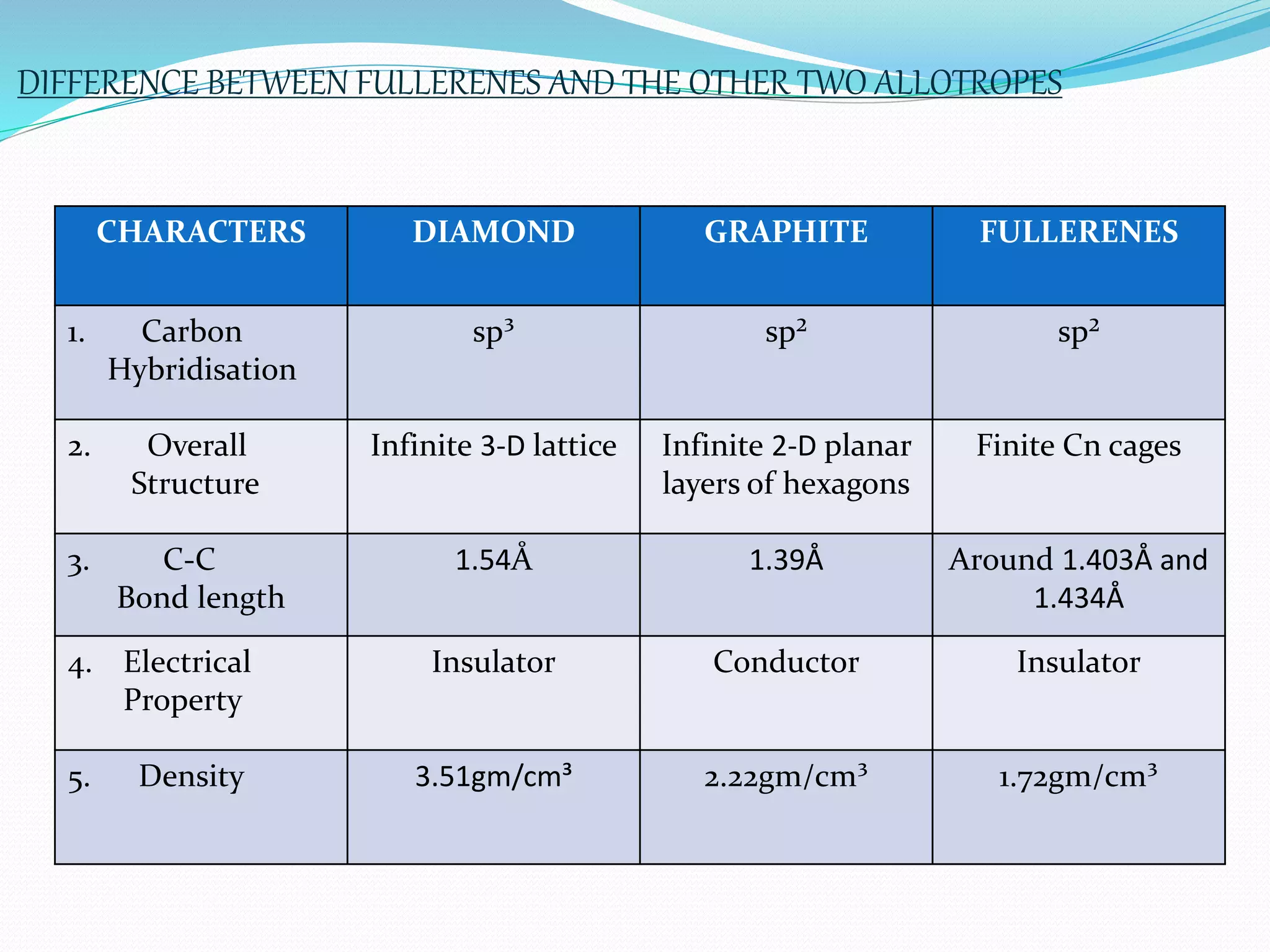
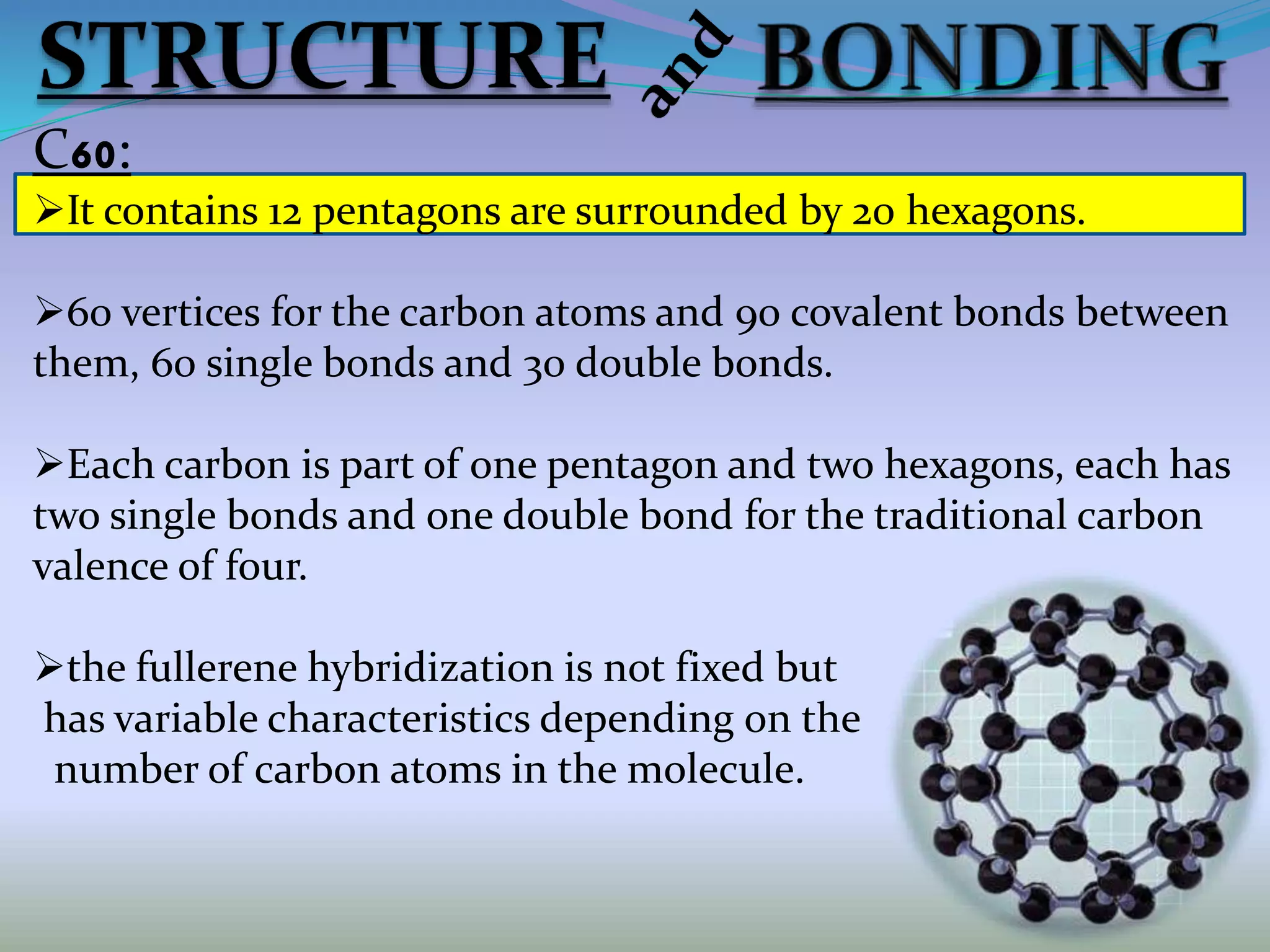
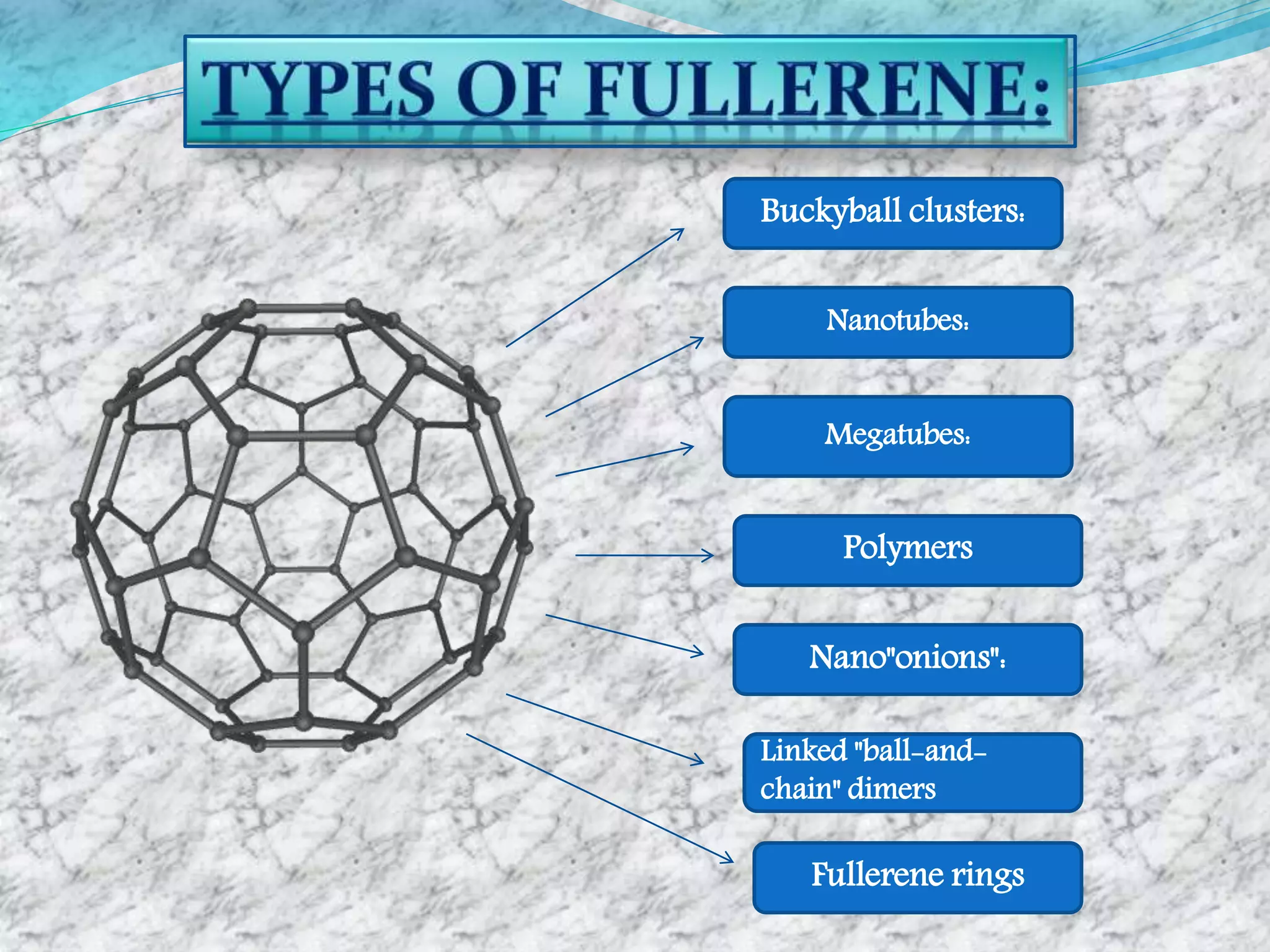
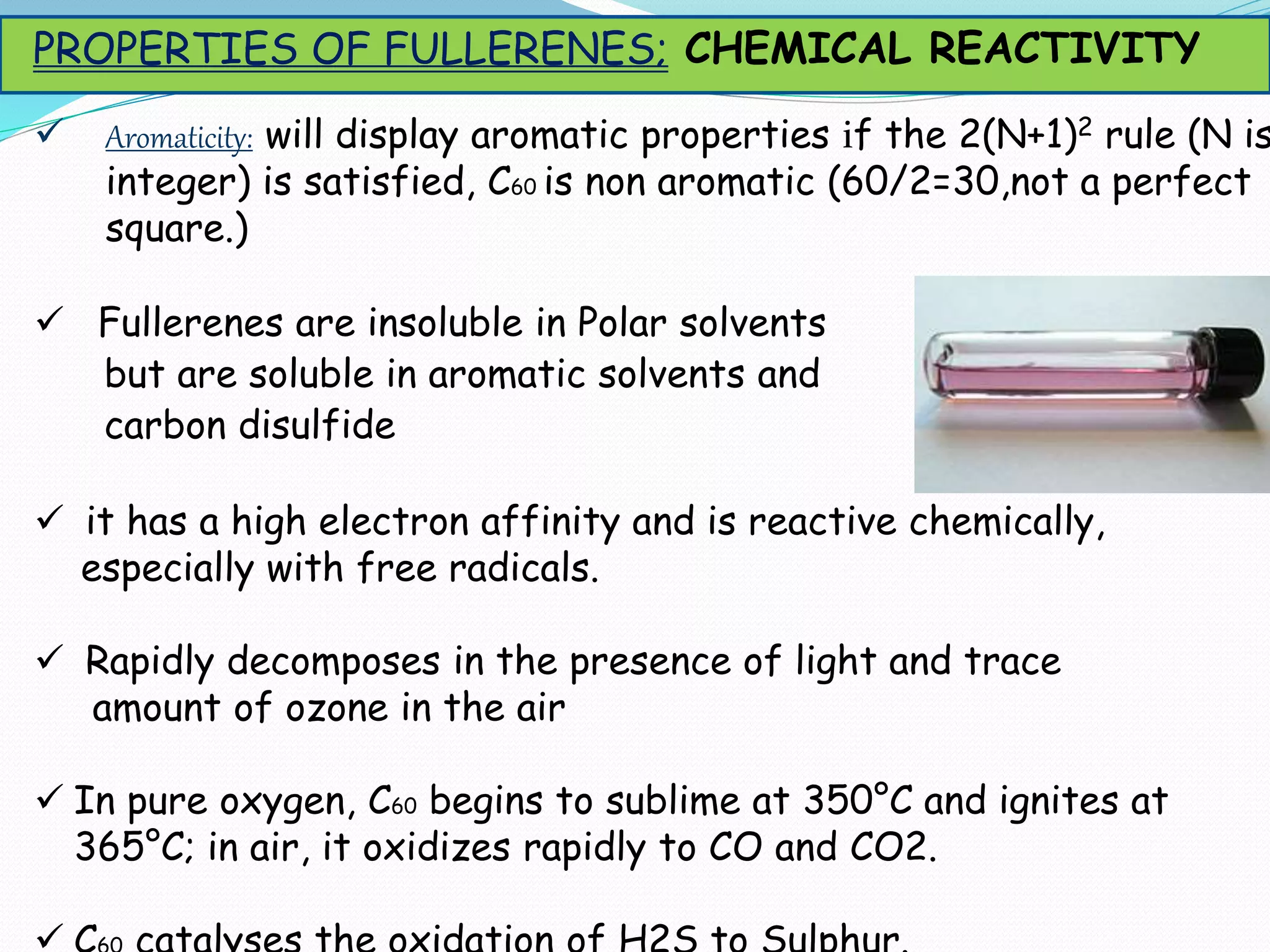
Fullerenes are allotropes of carbon consisting of hollow cages made of pentagonal and hexagonal rings. C60 buckminsterfullerene is the most famous fullerene, consisting of 60 carbon atoms arranged in 20 hexagons and 12 pentagons. Fullerenes were discovered in 1985 and have a variety of applications including use as antioxidants, in drug delivery, hydrogen storage, and more. They can form complexes with metals and be intercalated with other elements.















![.
The first use of fullerenes as ligands was when C60
was used as a ligand on platinum in the system
[(Ph3)P]2Pt(η2-C60)]
. Fullerene ligands behave similarly
to electron deficient alkenes and coordinate in
a dihapto fashion. This binding occurs on the
junction points of two 6-membered rings.
Higher hapticity is observed complexes of C60Ph5
−. In this system, bonding to
one of the 5-membered acts like a cyclopentadienyl ligand with
multiple substituents](https://image.slidesharecdn.com/fullerenesppt-180525181919/75/Fullerenes-ppt-22-2048.jpg)
![In other cases, a fullerene can bind
to multiple metal centres.
e.g [(Ph3P)2Pt]6C60
Uses:
Although no application has been commercialized, fullerene complexes are of
interest as potential catalysts, non-linear optical (NLO) materials, and as
supramolecular building blocks.
In this example, the complex has
binding very similar to ferrocene.](https://image.slidesharecdn.com/fullerenesppt-180525181919/75/Fullerenes-ppt-23-2048.jpg)



![• Hugh O. Pierson,HANDBOOK OF CARBON,GRAPHITE, DIAMOND AND
FULLERENES Properties, Processing and Applications.
• Seema Thakral* and R. M. Mehta, Fullerenes: An Introduction and Overview of
Their Biological Properties, Indian Journal of Pharmaceutical Sciences,2006
•S. Sivakamasundari, FULLERENES,ISBN 81-237-3958-3
•http://www.ch.ic.ac.uk/local/projects/Lertpibulpanya/k.html
•https://www.acs.org/content/acs/en/education/whatischemistry/landmarks/
fullerenes.html#discovery-of-fullerenes
•https://www.britannica.com/science/fullerene
•https://en.m.wikipedia.org/wiki/Fullerene
•B.C. Yadav and Ritesh Kumar, "Structure, properties and applications of fullerene",
International Journal of Nanotechnology and Applications
ISSN 0973-631X Volume 2, Number 1 (2008), pp. 15–24
•Maurizio Prato," [60]Fullerene chemistry for materials science applications",](https://image.slidesharecdn.com/fullerenesppt-180525181919/75/Fullerenes-ppt-27-2048.jpg)
