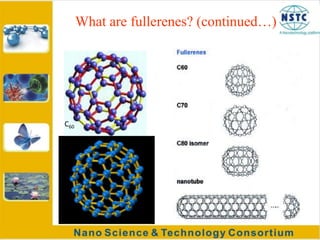
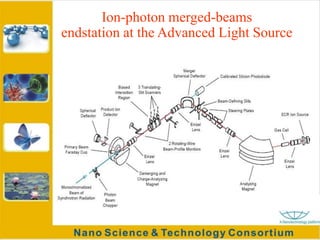
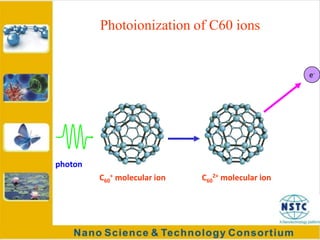
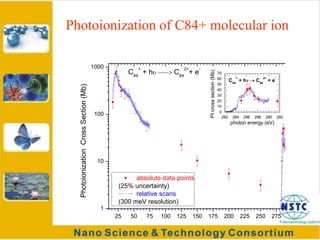
Fullerenes were discovered in 1985 at Rice University and consist of closed hollow cages of carbon atoms arranged in pentagonal and hexagonal rings. The most common fullerene is buckyball (C60), but others include C70, C72, etc. Fullerenes can be produced by vaporizing carbon in a gas medium and spontaneously forming in the condensing vapor. They are very stable due to their structure, with the highest tensile strength of any known material. Research shows fullerenes have applications as strong, resilient materials for armor and inhibiting HIV viruses due to antiviral properties when bonded to other elements.