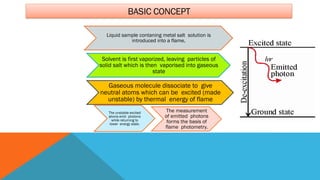
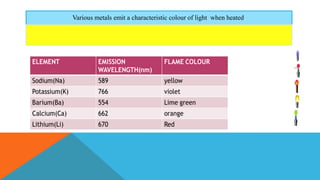
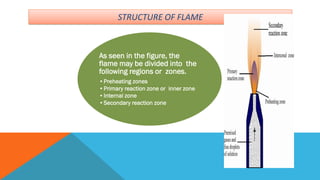
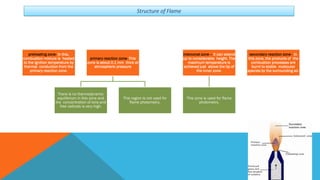
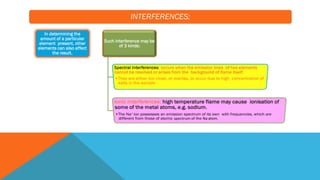
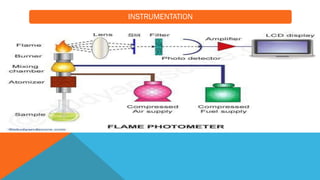
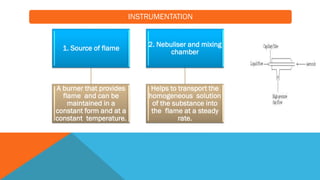
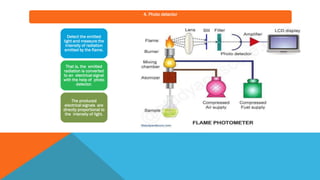
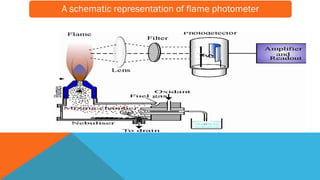
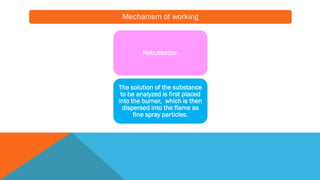
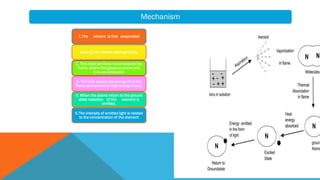
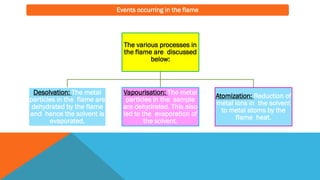
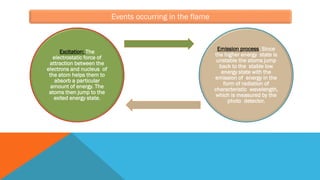
Flame photometry is a technique that uses the characteristic emissions of light from atoms excited in a flame to detect the presence of certain metal ions. When a sample containing metal ions is introduced into a flame, the metal ions are atomized and excited. As they return to lower energy states, they emit light of characteristic wavelengths. A photodetector measures the intensity of emitted light, which corresponds to the concentration of the metal ion in the original sample. Flame photometry is useful for quantitative analysis of metals like sodium, potassium, lithium, and calcium in applications such as clinical analysis of body fluids and agricultural soil testing.