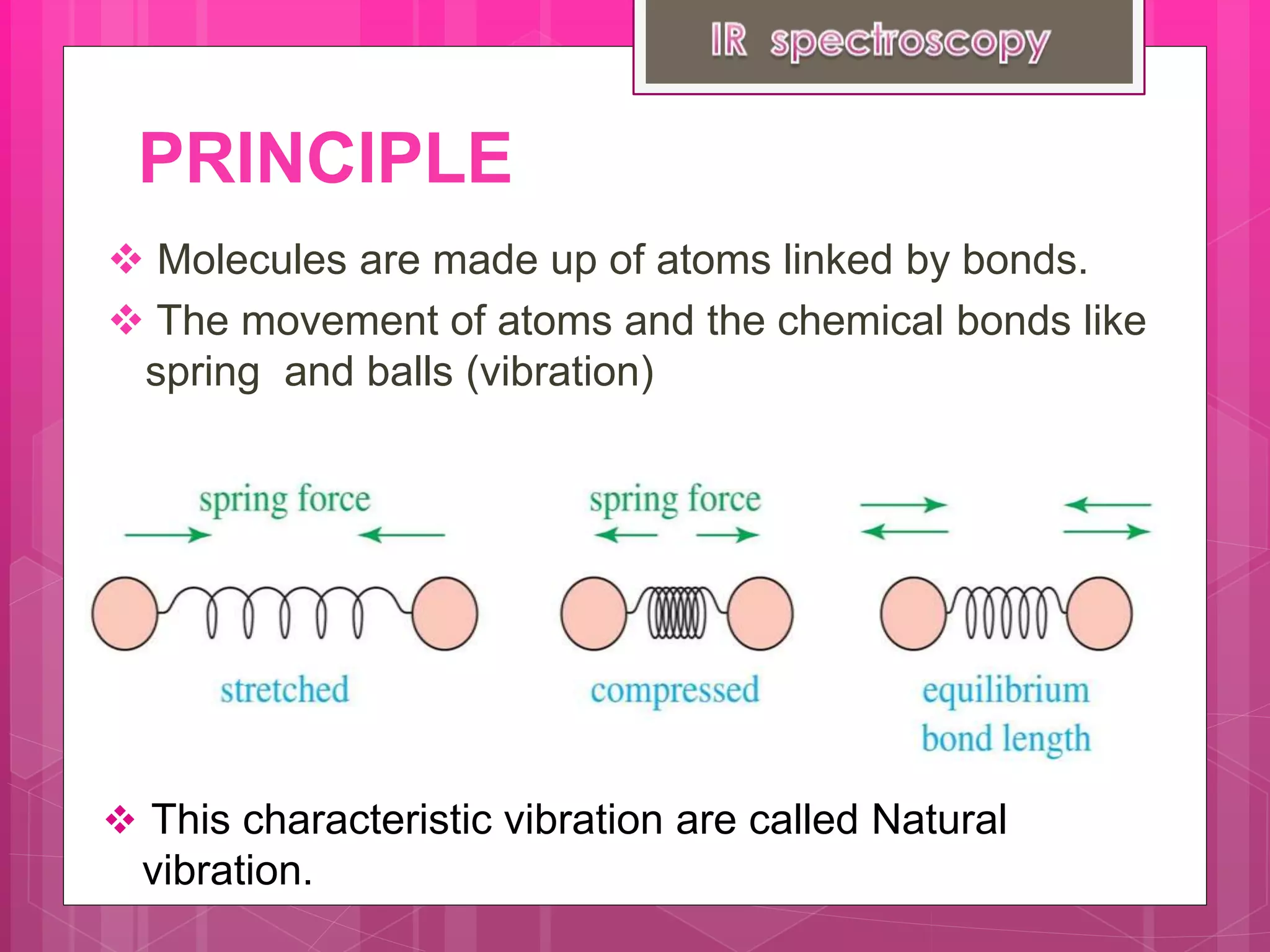
Infrared spectroscopy analyzes the absorption of infrared radiation by molecules to determine their structure. It works by exciting the vibrational modes of molecules, which correspond to characteristic absorption frequencies. The fingerprint region between 1500-500 cm-1 is especially useful for identifying functional groups and establishing molecular identity. Infrared spectrometers contain an infrared source, sample holder, detector, and recorder. Applications include identification of functional groups, structural elucidation of drugs and polymers, and quantitative analysis.