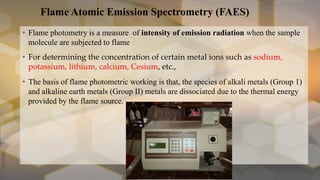
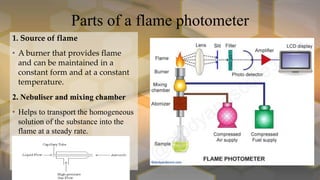
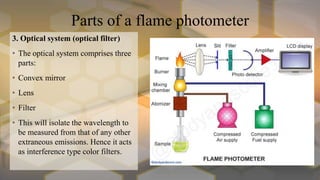
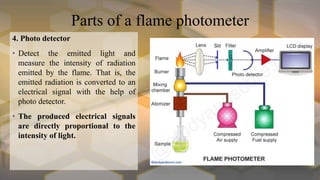
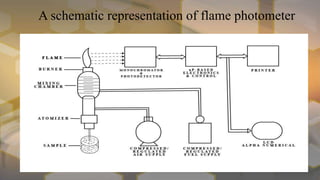
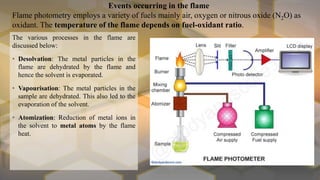
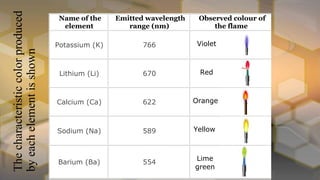
Flame photometry is an atomic emission spectroscopy technique used to measure the concentration of certain metal ions in solution by detecting the characteristic wavelengths of light emitted by their atoms when excited in a flame. The technique involves nebulizing the sample solution into a flame, where the solvent evaporates and the metal ions are atomized and excited to emit light. The intensity of the emitted light is measured by a photodetector and corresponds to the concentration of the metal ion. Common elements that can be analyzed using flame photometry include sodium, potassium, lithium, calcium, and cesium. The technique has applications in agriculture, clinical analysis, and food and beverage testing.