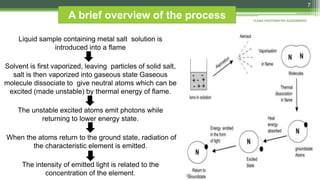
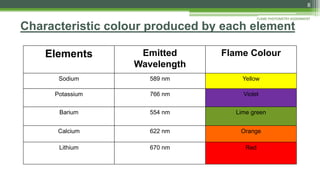
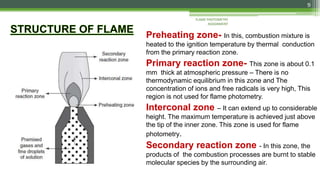
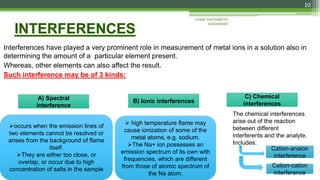
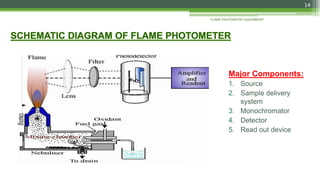
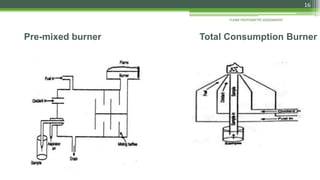
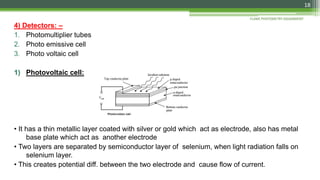
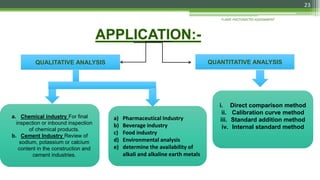
Flame photometry is a technique used to analyze sodium, potassium, lithium, calcium, and barium concentrations in solutions. It works by nebulizing a liquid sample into a flame, which excites the metal atoms. As the atoms return to the ground state, they emit light at characteristic wavelengths. A monochromator separates this light, which is measured with a detector. The light intensity is directly proportional to the metal's concentration. Interferences can occur from spectral overlap, ionization, or chemical reactions with other sample components. Applications include analyzing foods, beverages, pharmaceuticals, and more. Quantitative analysis is performed using calibration curves or standard addition methods.