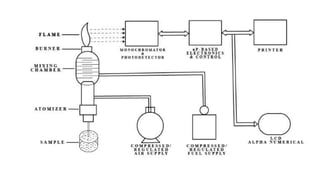
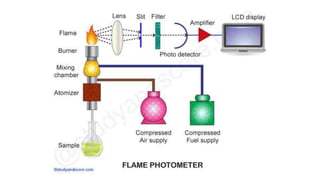
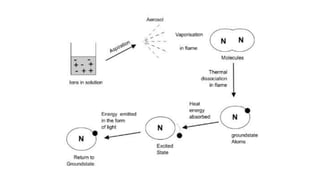
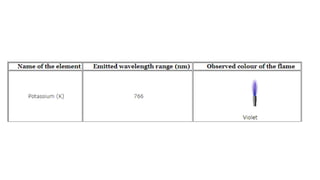
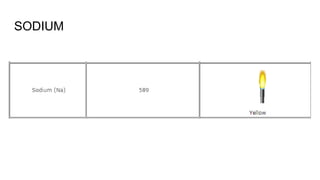
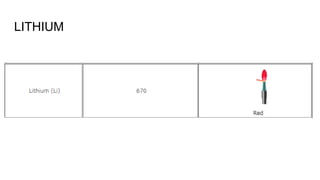
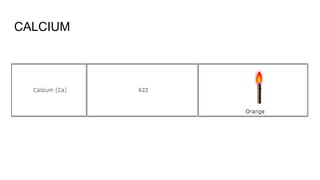
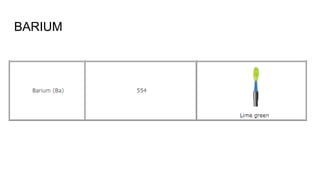
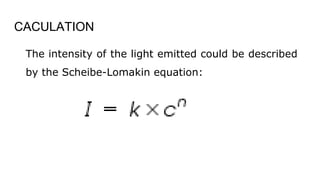Flame photometry is a technique used in atomic spectroscopy to analyze the concentration of metal ions like sodium and potassium through flame emissions. The method involves several steps including nebulization, atomization, and emission measurement, utilizing a photo detector to quantify the light emitted. Flame photometry is cost-effective and rapid, but has limitations such as the need for standard solutions and challenges in measuring high concentrations.