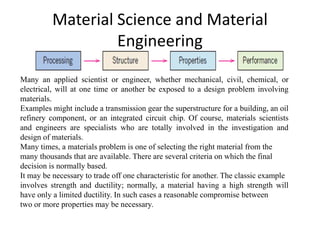
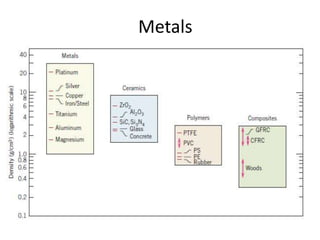
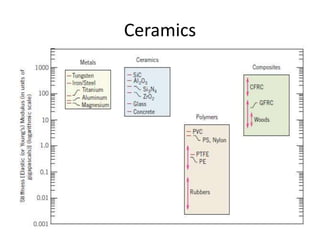
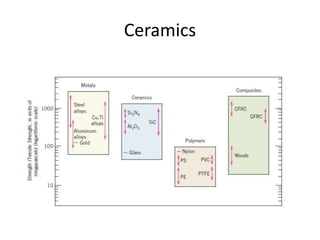
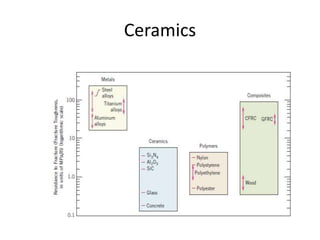
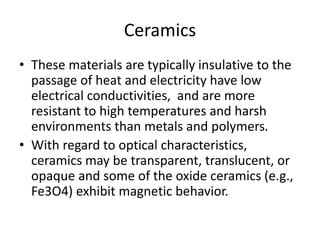
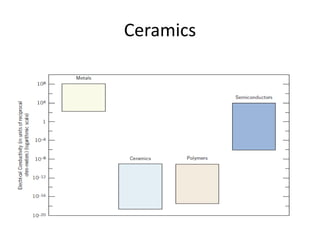
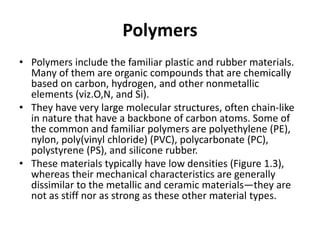
The document discusses the historical and contemporary roles of materials in society, emphasizing the significance of materials science and engineering in advancing technology and meeting human needs. It classifies materials into three primary categories: metals, ceramics, and polymers, along with composites that combine these materials to achieve desired properties. The text highlights the evolution of materials development and the relationship between their structure and properties, illustrating how this knowledge has led to innovative applications in various fields.