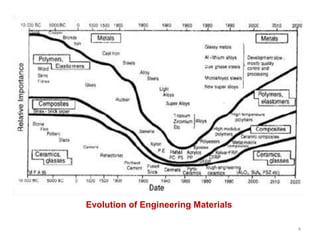
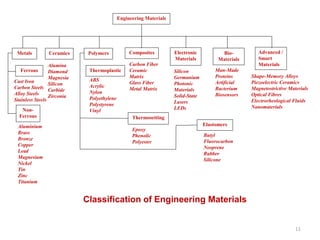
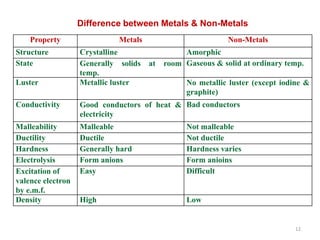
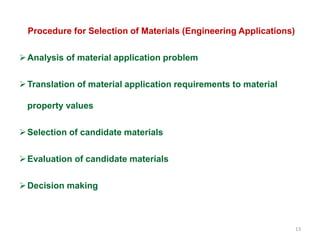
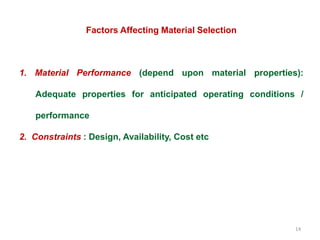
The document provides an overview of engineering materials, discussing their evolution from natural materials in the Stone Age to advanced smart materials used today. It outlines the significance of material science and engineering for various fields, including mechanical, electrical, and aerospace engineering, alongside a classification system based on general properties, nature, and applications. Key factors influencing material selection are presented, along with their essential properties and the importance of understanding the relationships between structure, properties, and processing.