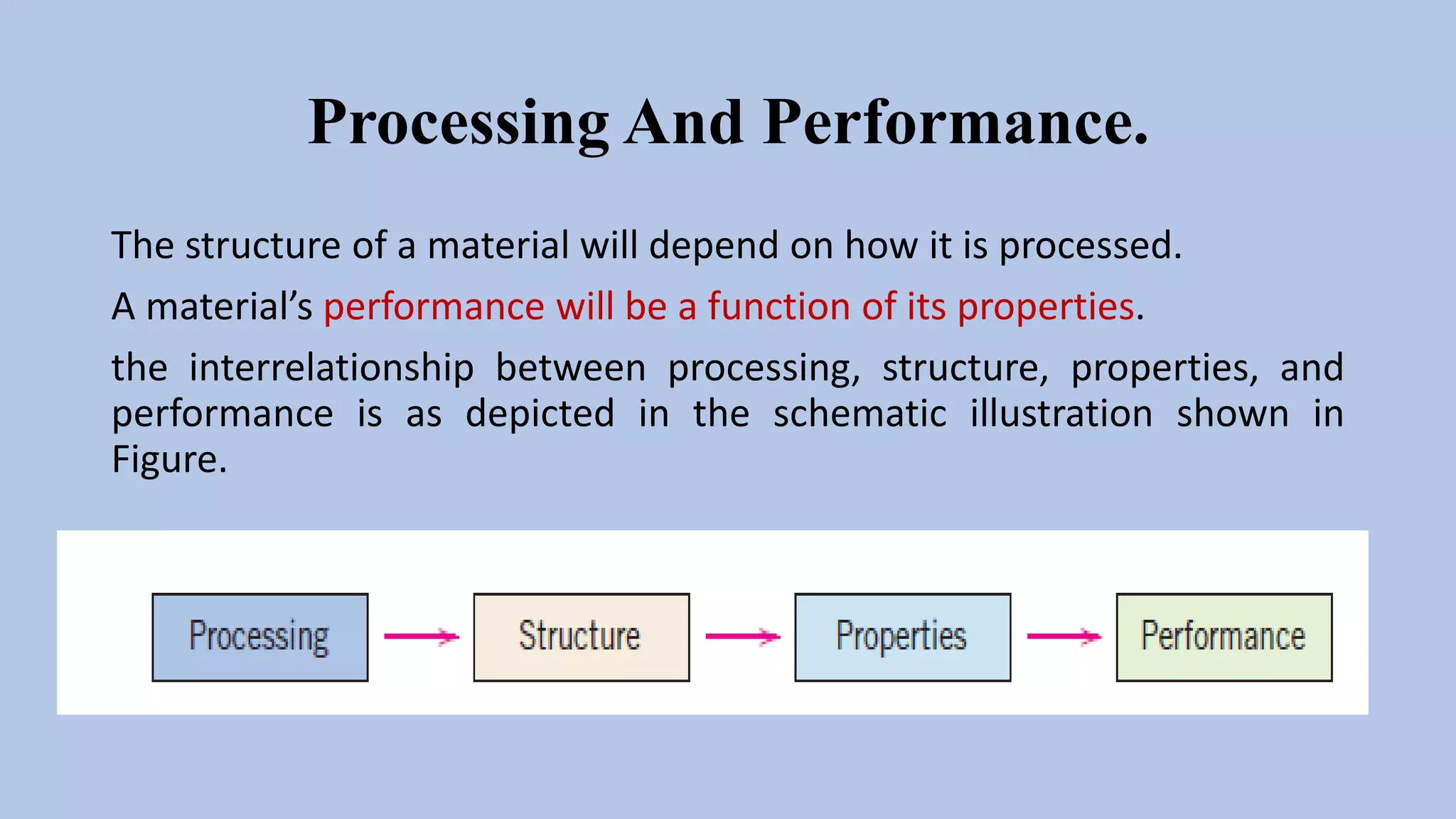
The document discusses the relationships between processing, structure, properties, and performance of materials, highlighting how the structure affects optical properties in examples of aluminum oxide specimens. It emphasizes the importance of materials science and engineering in selecting appropriate materials based on in-service conditions, property characteristics, and economic considerations. The document classifies materials into four categories: metals, ceramics, polymers, and composites, detailing their distinct properties and applications.