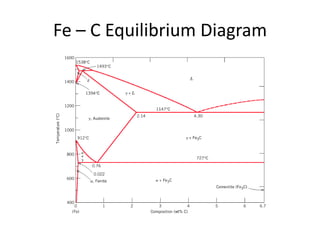
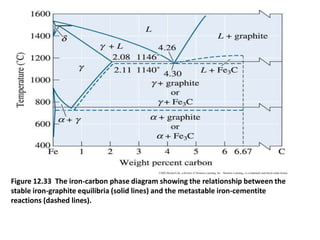
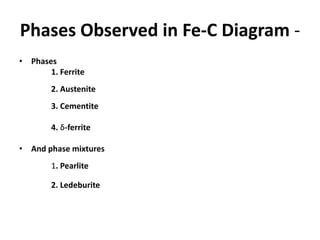
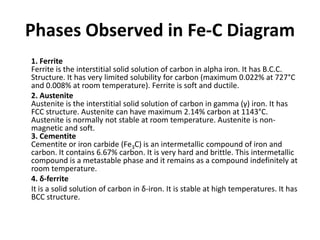
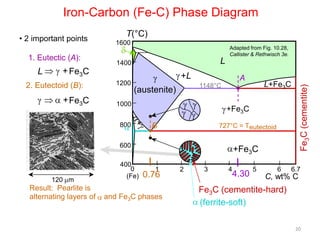
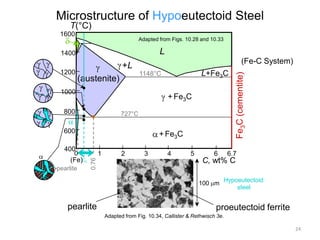
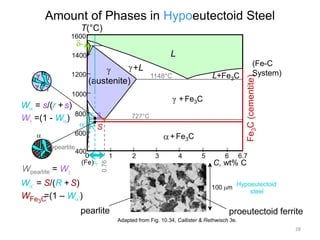
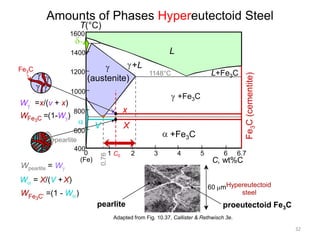
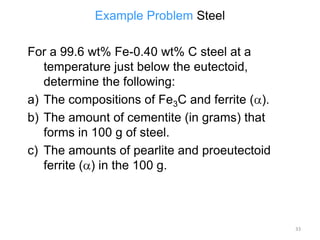
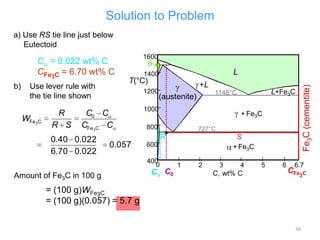
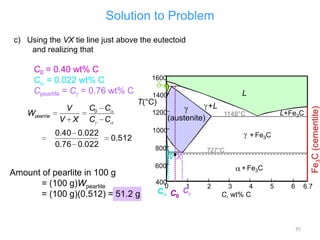
The document discusses the iron-carbon phase diagram. It describes three important reactions:
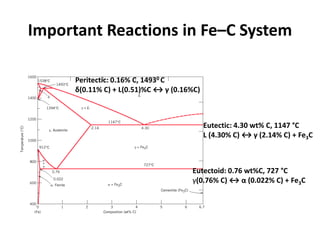
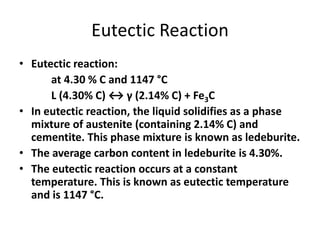
1) The eutectic reaction occurs at 4.3% carbon and 1,147°C, where liquid transforms to austenite and cementite.
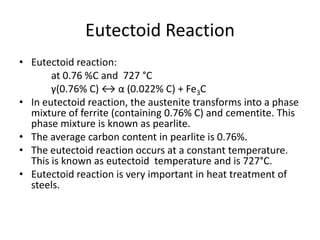
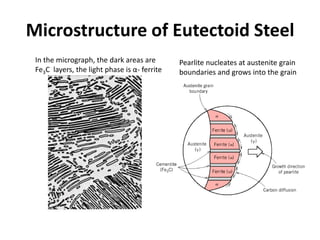
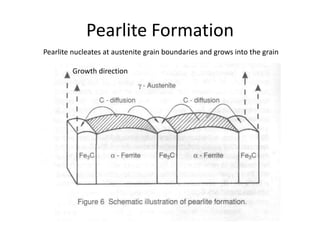
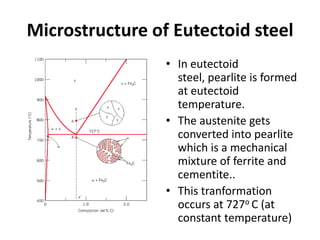
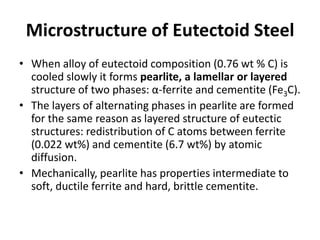
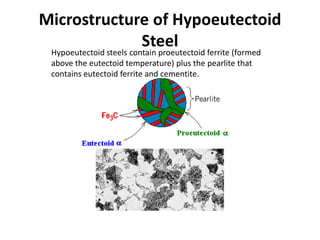
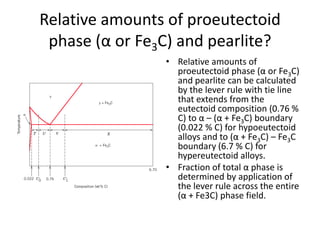
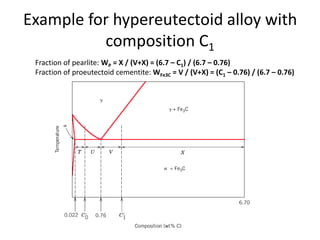
2) The eutectoid reaction occurs at 0.76% carbon and 727°C, where austenite transforms to ferrite and cementite to form pearlite.
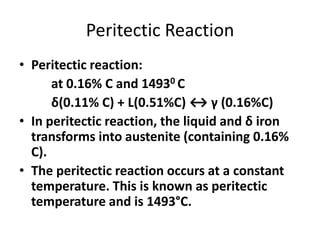
3) The peritectic reaction occurs at 0.16% carbon and 1,493°C, where liquid and delta-ferrite transform to austenite.
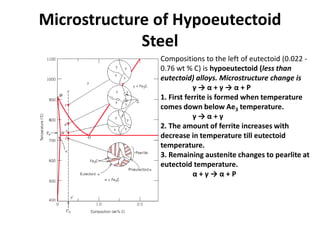
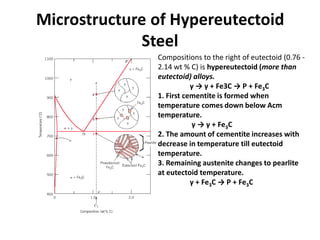
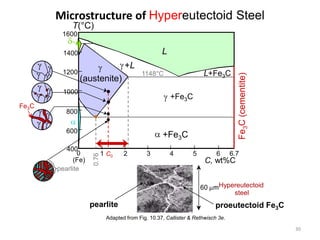
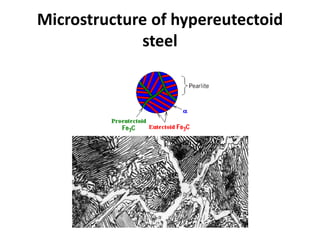
The phase diagram is used to explain the microstructures that form in steels with different carbon