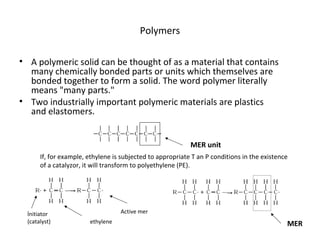
The document provides an overview of materials science, which explores the relationship between material structure and properties, and its historical significance in civilization. It classifies engineering materials including metals, ceramics, polymers, and composites, detailing their properties and applications. Additionally, the document highlights the importance of understanding materials for engineering disciplines and innovation.