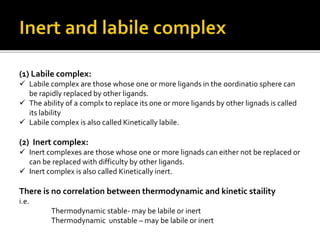
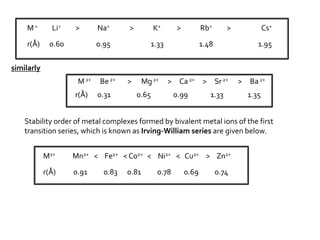
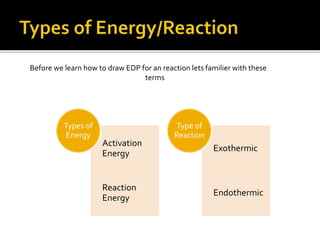
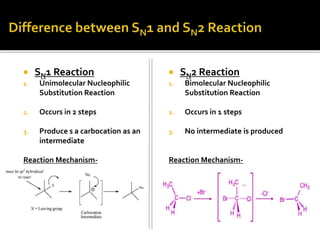
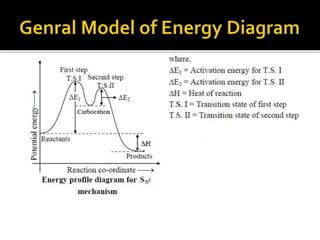
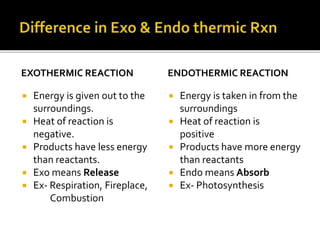
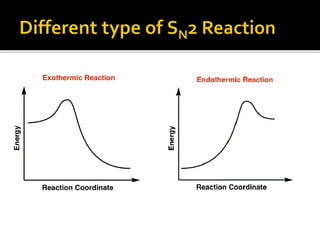
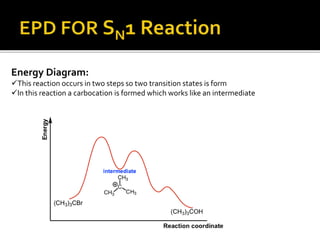
This document discusses energy diagrams and types of stability in chemistry. It begins with an introduction to energy diagrams and how they are used to represent energy changes during chemical reactions. It then discusses different types of reactions like SN1 and SN2 and how their energy profiles differ based on reaction mechanism. It also defines terms like activation energy, reaction energy, exothermic and endothermic reactions. The document goes on to explain kinetic and thermodynamic stability of complexes and factors that influence the stability of metal complexes like size and charge of the metal ion, basicity of ligands, and solvent effects.





![If we say this complex is stable or not , our means is to how much time we can
store that complex in nature and this complex doesnot oxidise or reduct.
But it is ready to acknowledge one complex which is stable for some condition
It may be unstable for another condition
For example: [Cu(NH3)4]SO4
is an stable substance, we can store for a long time in
in solid state but when we put it in acidic aquous solution this substract is
reduct in very short period of time.](https://image.slidesharecdn.com/mt2rkxfkttuuc8jgb8fd-energy-profile-diag-and-stability-aashutosh-sem1-230201075950-178c2dda/85/Energy-Profile-Diagram-and-Stability-12-320.jpg)