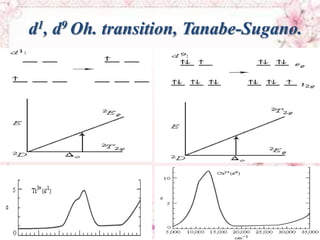
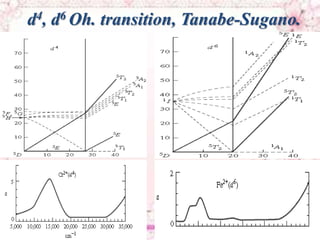
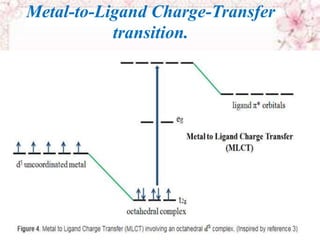
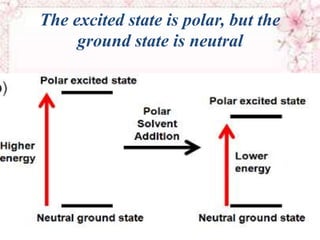
This document discusses electronic spectra of metal complexes. It begins by defining quantum numbers related to electron configuration, such as L (total orbital angular momentum) and l (secondary quantum number). It then describes two main types of electronic transitions in coordination compounds: d-d transitions specific to metals, and charge-transfer transitions. The remainder of the document discusses charge-transfer transitions in more detail, defining ligand-to-metal and metal-to-ligand charge transfer, and how solvent polarity affects these transitions.