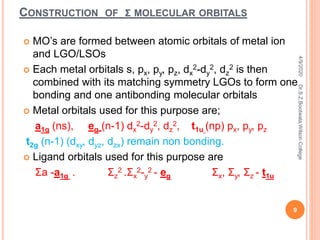
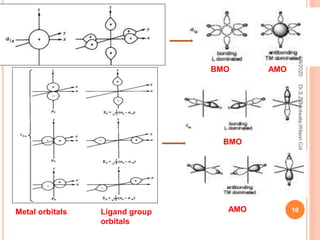
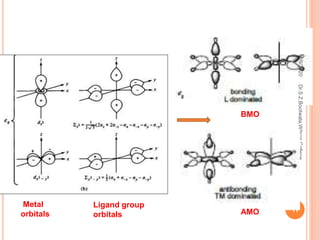
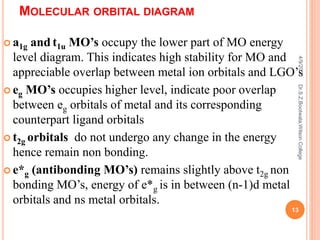
Molecular orbital theory describes how atomic orbitals of metal ions and ligand group orbitals interact to form molecular orbitals. For interaction to occur, the atomic orbitals must have similar energies, symmetry, and overlap appreciably. Molecular orbitals are formed through sigma and pi bonding. Sigma bonding results from the combination of metal orbitals along the bonding axis with ligand group orbitals of the same symmetry. Pi bonding occurs through overlap of metal ion t2g orbitals with ligand pi orbitals. The type of pi bonding that results depends on whether the ligand pi orbitals are filled or empty.




![ In dx
2-y
2 metal orbital ,one opposite pair of lobes has
positive sign and other has negative sign ,the liner
combination of ligands orbital to form LGO is shown as
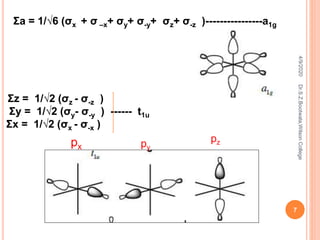
Ʃx2-y2 = 1/2 [( σx + σ –x) – ( σy+ σ-y)]
The formation of LGOs which can effectively overlap dz
2
metal orbital which is located on z-axis can be shown as
Ʃz2
Ʃz2 = 1/ √12 ( 2 σz+ 2σ-z - σx - σ –x- σy- σ-y)
4/9/2020
8
Dr.S.Z.Bootwala,WilsonCollege](https://image.slidesharecdn.com/molecularorbitaltheory-200409114311/85/Molecular-orbital-theory-8-320.jpg)


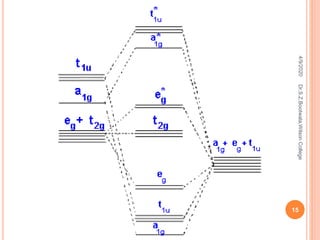
![HEXAAQUO TITANIUM (III) ION [TI(H2O)6]3+
Ti (At. No. 22) -3d2 4s2 ,Ti3+ 3d1 4s0
Number of electrons in d orbital of Ti3+ = 1
Number of electrons of 6 ligands = 12
Total electrons to be filled = 13
Out of the 13 electrons, the first 12
electrons will be placed in the lower
energy six bonding MO’s. a1g t1u and eg.
These orbitals have more ligand
character.
The remaining 1 electron occupies non
bonding t2g orbital. which explains their
weak paramagnetic nature of the
complex.
A single band in the absorption spectrum
of this complex is attributed to a transition
from triply degenerate t2g ground state to
doubly degenerate excited e*g state.
4/9/2020
16
Dr.S.Z.Bootwala,WilsonCollege](https://image.slidesharecdn.com/molecularorbitaltheory-200409114311/85/Molecular-orbital-theory-16-320.jpg)
![HEXAFLUORO FERRATE (III) ION [FE (F)6]3-
Fe (At. No. 26) 3d6 4s2
Fe3+ 3d5 4s0 4p0
Number of electrons in d orbital of Fe3+=
5
Number of electrons of 6 ligands = 12
Total electrons to be filled = 17
Out of the 17 electrons, the first 12
electrons will be placed in the lower
energy six bonding MO’s. a1g t1u and eg.
These orbitals have more ligand
character.
The remaining 5 electrons occupies non
bonding t2g orbital and antibonding e*g
orbitals.
Thus in this complex, 5 unpaired
electrons are present. Therefore this
complex is highly paramagnetic and it’s
a high spin complex.
4/9/2020
17
Dr.S.Z.Bootwala,WilsonCollege](https://image.slidesharecdn.com/molecularorbitaltheory-200409114311/85/Molecular-orbital-theory-17-320.jpg)
![HEXACYANO FERRATE (III) ION [FE(CN)6]4-
Fe (At. No. 26) 3d6 4s2
Fe2+ -3d6 4s0 4p0
Number of electrons in d orbital of Fe2+ = 6
Number of electrons of 6 ligands = 12
Total electrons to be filled = 18
Out of the 18 electrons, the first 12 electrons
will be placed in the lower energy six
bonding MO’s. a1g t1u and eg. These orbitals
have more ligand character. The remaining 6
electrons occupy non bonding t2g orbital.
The electron prefer to pair up in t2g rather
than occupying e*g orbital, as the energy gap
between t2g and e*g orbital is large. This
higher value of Δo is credited to the greater
extent of overlap between eg orbitals of
metal and ligand orbitals.
Thus in this complex all electrons are
paired. Hence it is diamagnetic and a low
spin complex.
4/9/2020
18
Dr.S.Z.Bootwala,WilsonCollege](https://image.slidesharecdn.com/molecularorbitaltheory-200409114311/85/Molecular-orbital-theory-18-320.jpg)
![MOLECULAR DIAGRAM OF THE FOLLOWING
Hexacyano ferrate (III) ion [Fe(CN)6]3-
Hexafluoro ferrate (II) ion [Fe(F)6]4-
Hexafluoro cobaltate (III) ion [Co(F)6]3-
Hexammine cobalt (III) ion [Co(NH3)6]3+
4/9/2020
19
Dr.S.Z.Bootwala,WilsonCollege](https://image.slidesharecdn.com/molecularorbitaltheory-200409114311/85/Molecular-orbital-theory-19-320.jpg)

![A)EFFECT OF FILLED Π ORBITALS ( L → MΠ-INTERACTION)
In an octahedral complex like
[Fe(F)6]3- the F- ion have π
orbitals as well as σ orbitals.
The σ interaction takes place as
shown earlier.
As fluorine atom is
electronegative, the π orbitals
will be at a low energy level
than the corresponding t2g metal
orbitals.
Under these conditions, the
bonding π orbitals ( t2g
(b) ) will
be nearer to ligand symmetry
orbitals used for π bonding.
4/9/2020
22
Dr.S.Z.Bootwala,WilsonCollege](https://image.slidesharecdn.com/molecularorbitaltheory-200409114311/85/Molecular-orbital-theory-22-320.jpg)
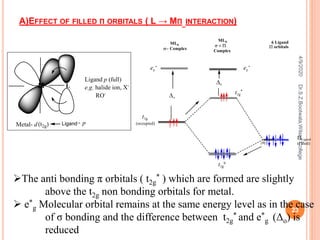
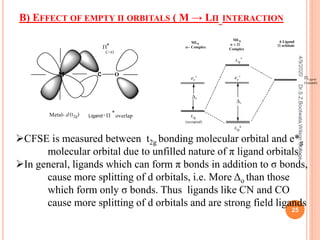
![B) EFFECT OF EMPTY Π ORBITALS ( M → LΠ INTERACTION)
In an octahedral complex like
[Fe(CN)6]3- the cyanide ions have
vacant π orbitals in addition to σ
orbitals.
As the π orbitals of ligands are
empty, they are comparatively at
higher energy level than t2g orbitals
of metal ion.
The bonding and antibonding π
molecular orbitals are formed due to
π interactions, out of which t2g
*
molecular orbital occupies higher
energy level than e*
g , t2g bonding
molecular orbital however goes to
lower energy level at much more
stabilised Δo.
4/9/2020
24
Dr.S.Z.Bootwala,WilsonCollege](https://image.slidesharecdn.com/molecularorbitaltheory-200409114311/85/Molecular-orbital-theory-24-320.jpg)