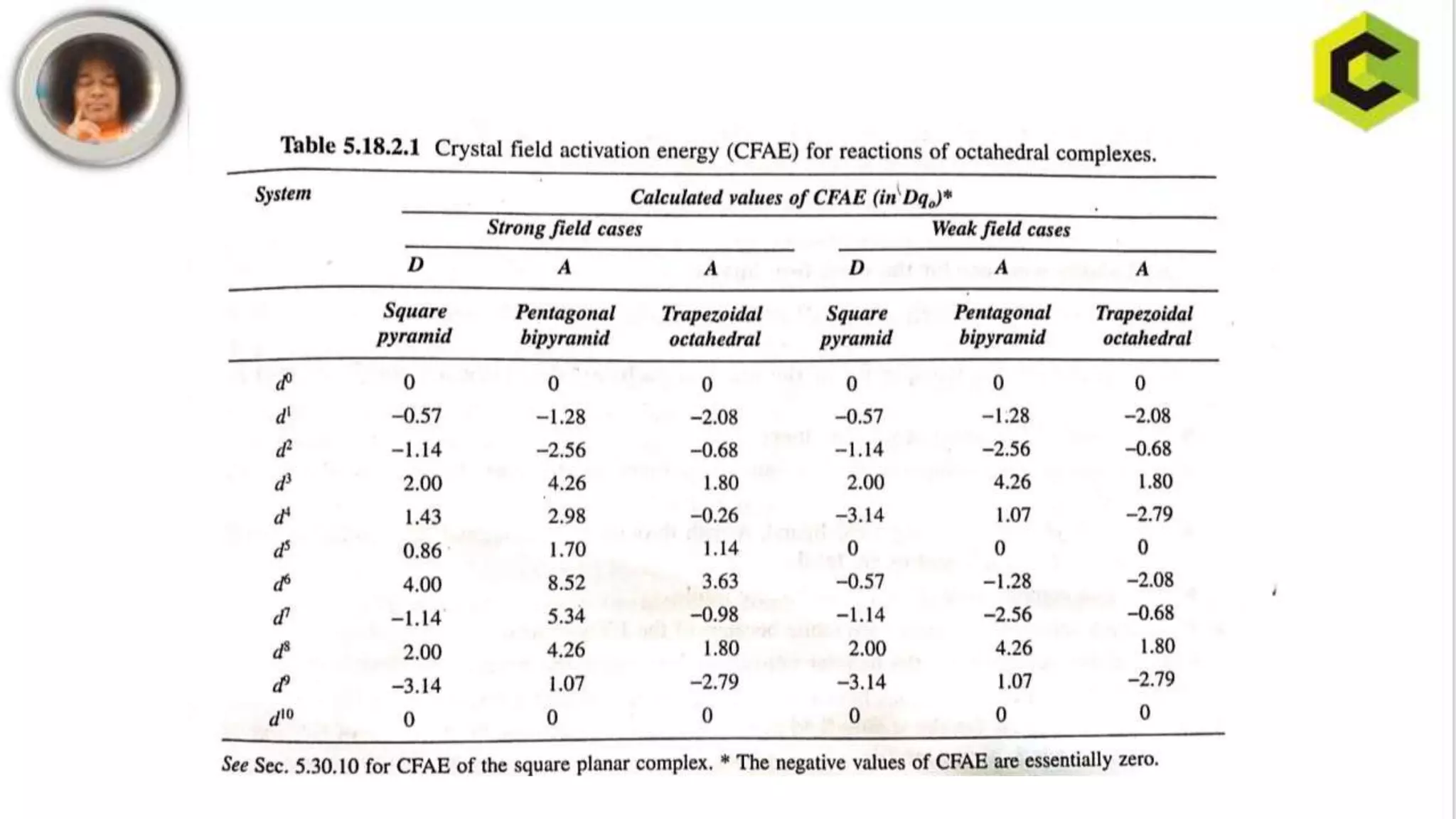
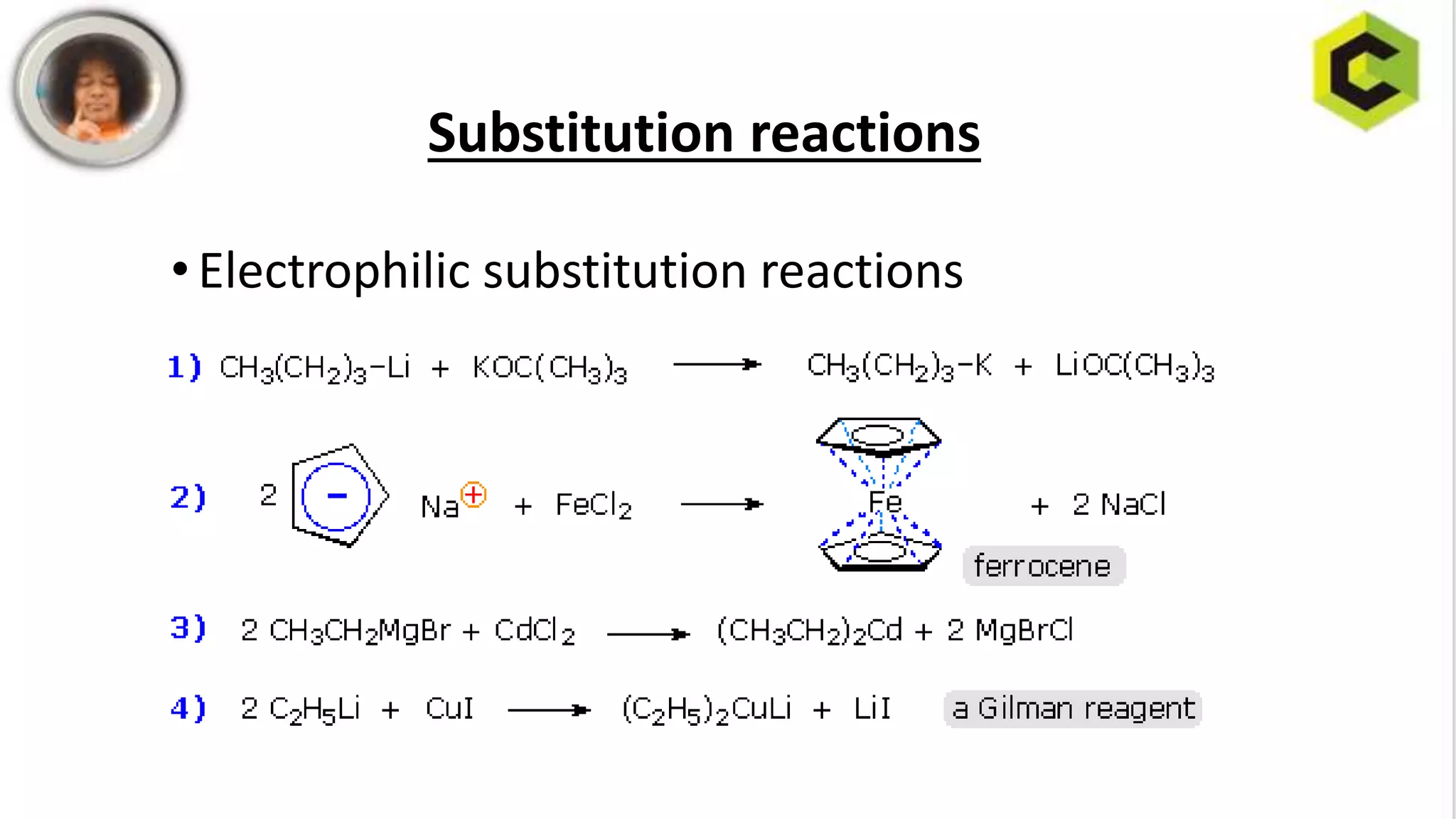
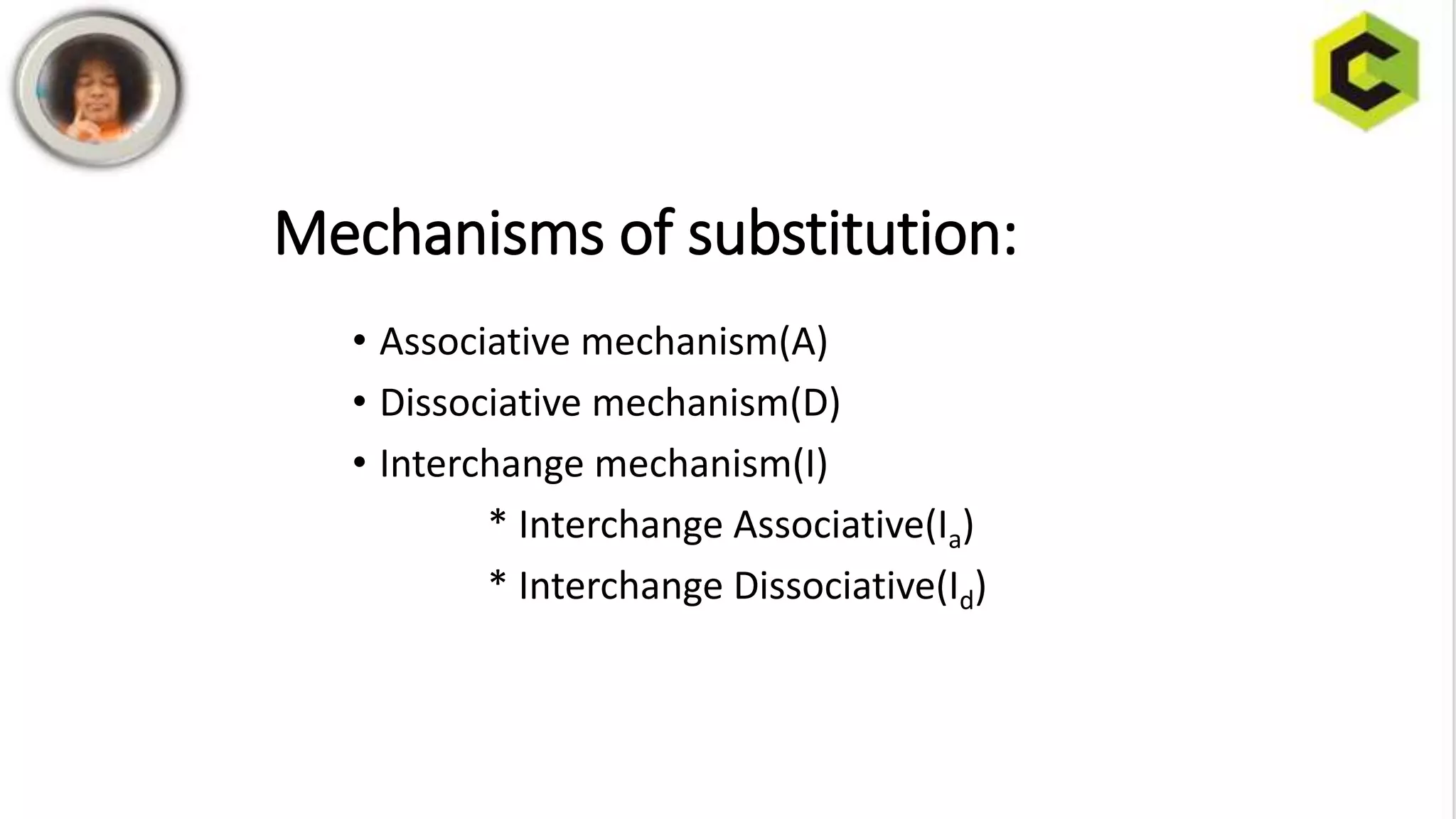
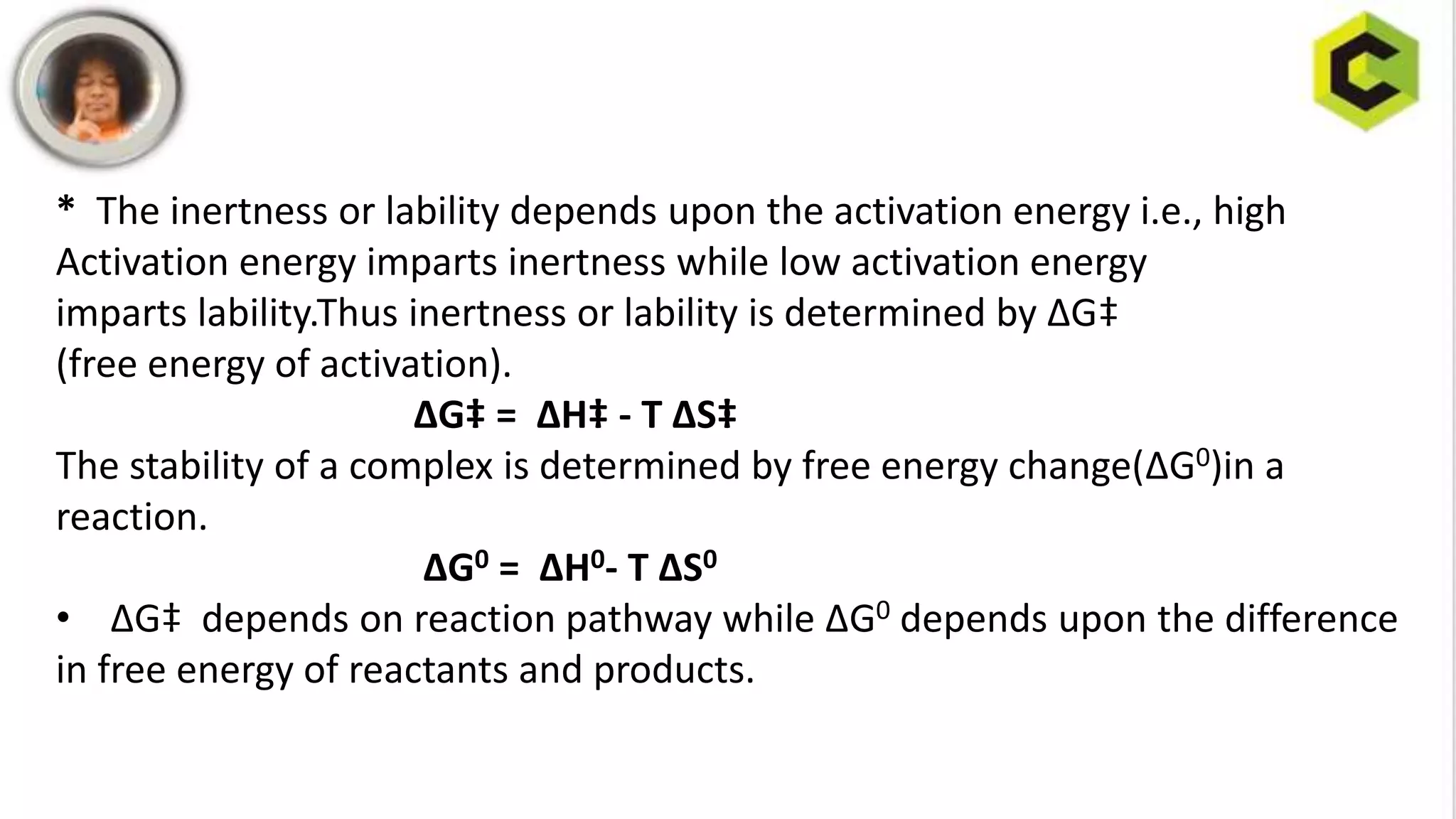
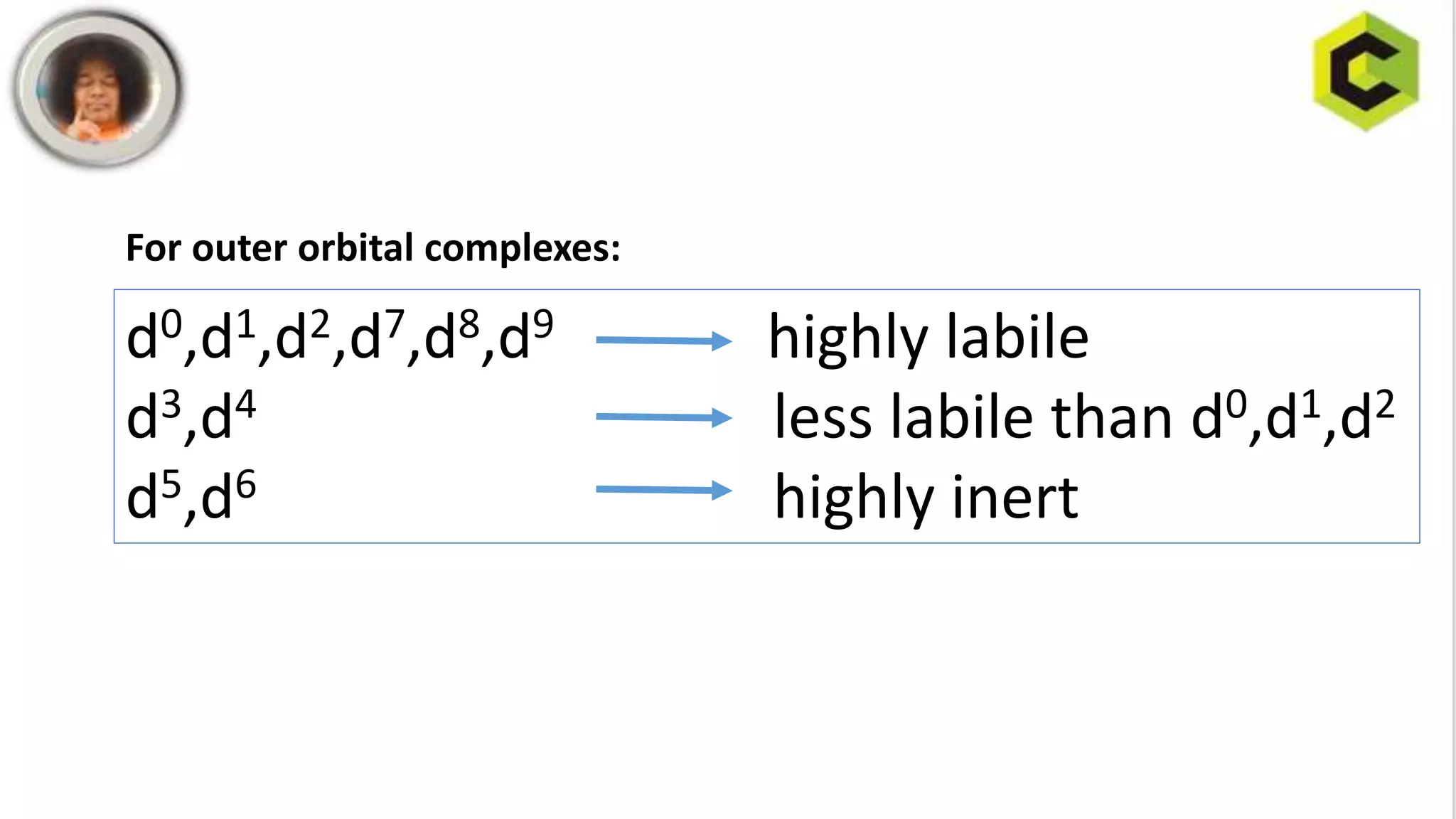
This document discusses substitution reactions and their mechanisms in coordination complexes. There are two main types of substitution reactions: electrophilic and nucleophilic. Nucleophilic substitution can occur through acid hydrolysis, base hydrolysis, and ligand exchange reactions. The main mechanisms of substitution are associative, dissociative, and interchange mechanisms. Lability and inertness of complexes depends on both kinetic and thermodynamic factors. According to valence bond theory, the electronic configuration of the metal center determines if a complex is labile or inert. Crystal field theory also aims to explain lability and inertness based on crystal field stabilization energies. Square planar complexes can exhibit substitution governed by trans influence effects.


![slow +Y(fast)
[L5MX] [L5M] [L5MY]
5-coordinate complex
slow X -X(fast)
[L5MX]+Y [L5M ] [L5MY] + X
Y
7-coordinate complex
slow X -X(fast)
[L5MX]+Y [L5M ] [L5MY] + X
Y
7-coordinate transition state
Associative :
Dissociative :
Interchange :](https://image.slidesharecdn.com/inertandlabilecomplexesandsubstitutionreactions-190831072953/75/Inert-and-labile-complexes-and-substitution-reactions-5-2048.jpg)




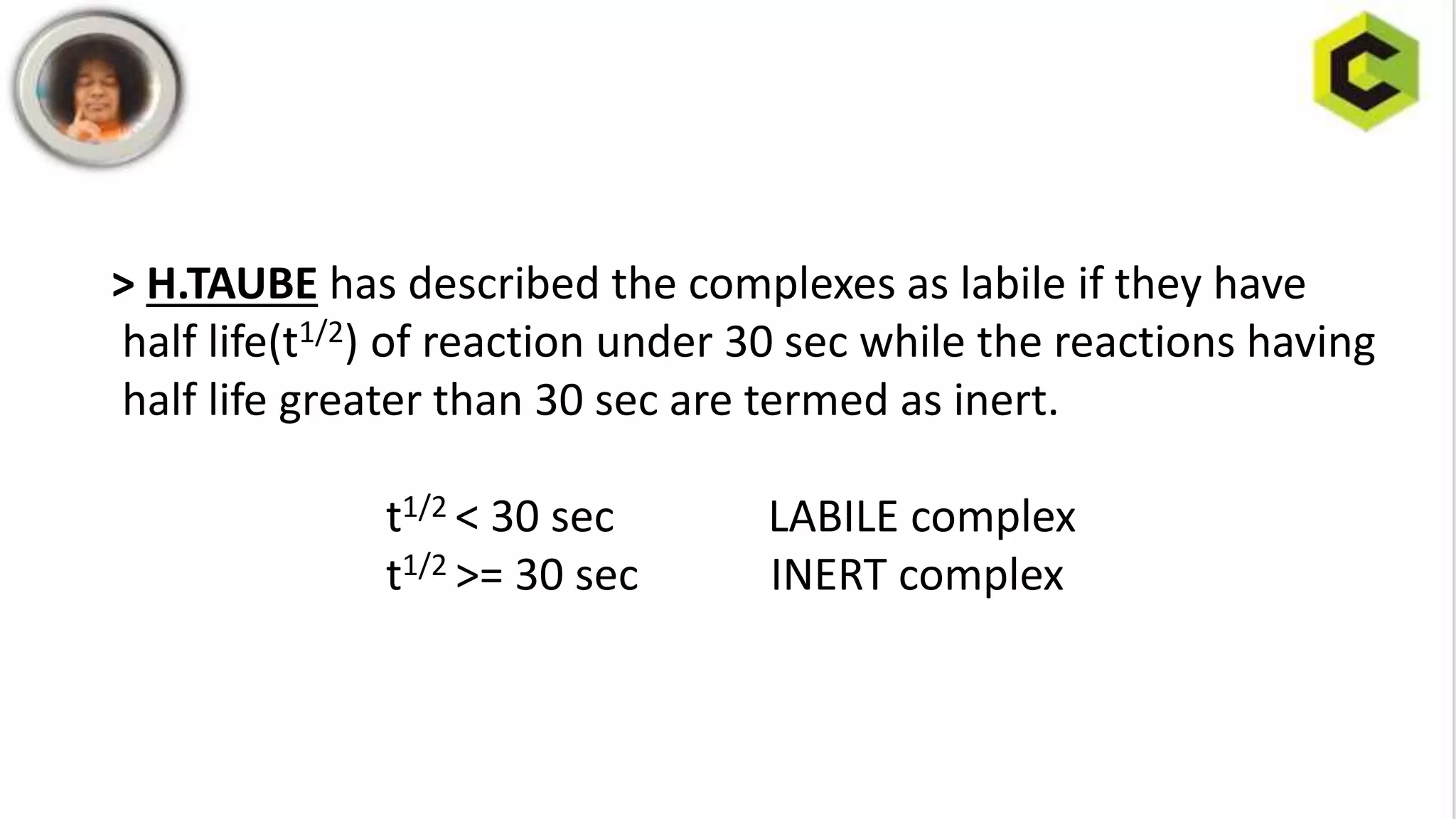
![The terms inert and labile are kinetic terms because they reflect the rate with
which the reaction proceeds and these kinetic terms should not be confused
With thermodynamic terms stable and unstable which refer to tendency of
species to exist(governed by equilibrium constants.)
Consider the reaction: M + nL ⇌ MLn ; βn =[MLn]/[M][L]n
where βn is formation constant of the complex.
The higher values of βn indicate it’s higher thermodynamic stability of the
complex. Thus it gives measure of the extent to which the reaction proceeds
but it cannot say anything about the speed with which equilibrium is attained.](https://image.slidesharecdn.com/inertandlabilecomplexesandsubstitutionreactions-190831072953/75/Inert-and-labile-complexes-and-substitution-reactions-11-2048.jpg)
![Examples:
* [Hg(CN)4]-2 + 4 14CN- ⇌ [Hg14(CN)4]-2 + 4CN-
log βn =42 ; t1/2= very small
*[Cr(CN)6]-3 + 6 14CN- ⇌ [Cr14(CN)6]-3 + 6 CN-
log βn =37 ; t1/2= 24 days
Thermodynamically
Stable and kinetically
Unstable
Thermodynamically
Stable and kinetically
stable](https://image.slidesharecdn.com/inertandlabilecomplexesandsubstitutionreactions-190831072953/75/Inert-and-labile-complexes-and-substitution-reactions-12-2048.jpg)
![[V(NH3)6]+3:
[MnCl6]-3:
[Co(CN)6]-3:
(n-1)d
(n-1)d
(n-1)d
ns
ns
ns
np
np
np nd
d2sp3 hybridisation
vacancy for entering nucleophile](https://image.slidesharecdn.com/inertandlabilecomplexesandsubstitutionreactions-190831072953/75/Inert-and-labile-complexes-and-substitution-reactions-16-2048.jpg)