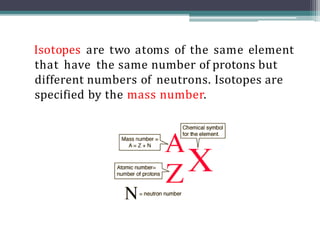
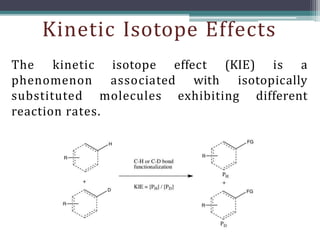
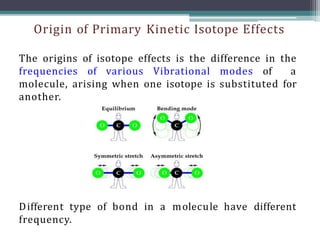
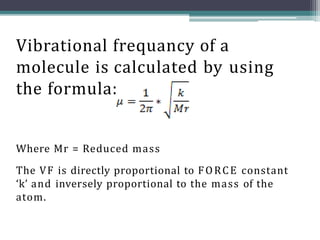
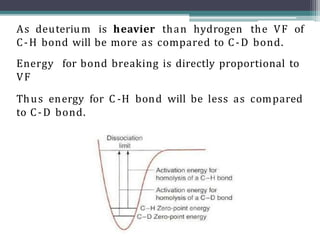
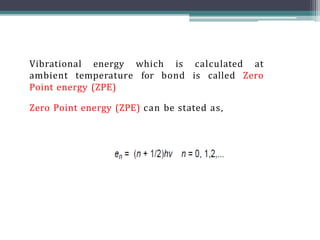
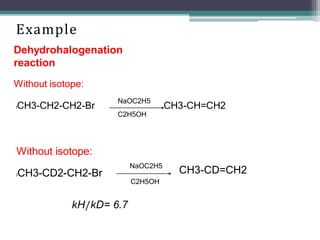
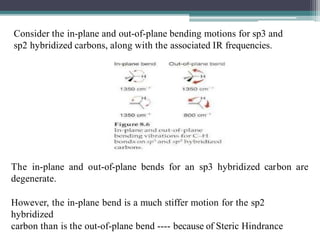
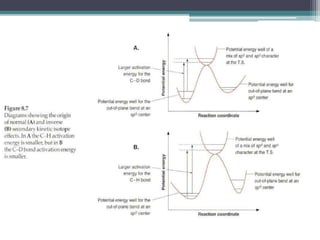
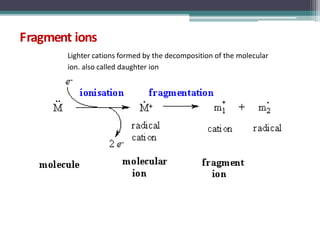
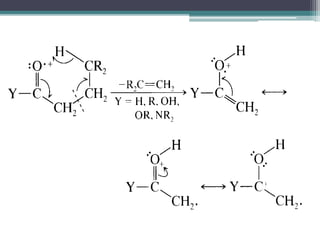
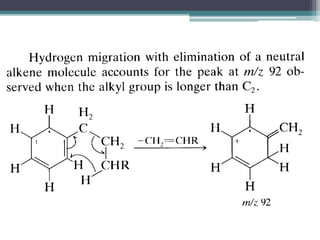
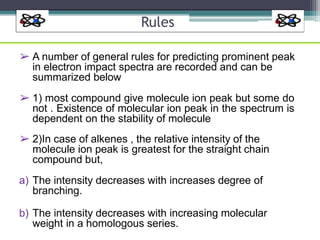
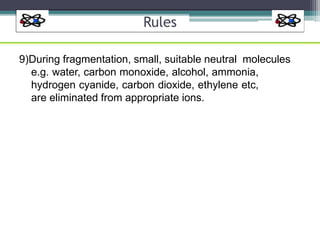
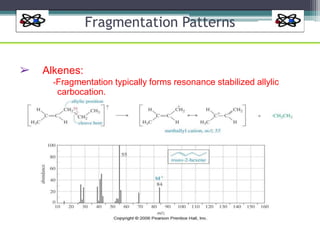
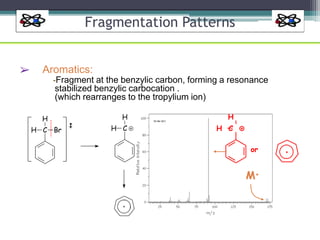
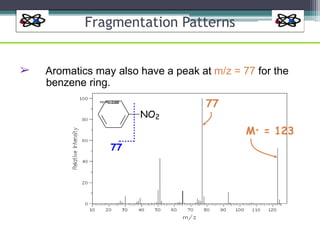
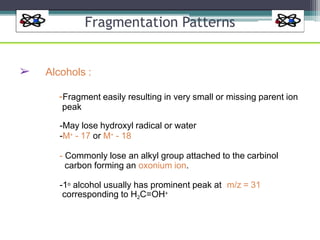
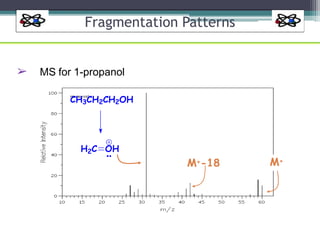
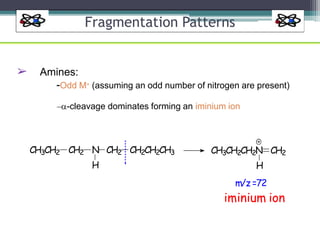
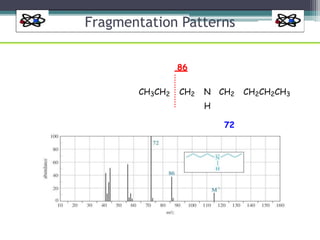
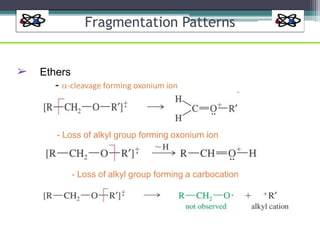
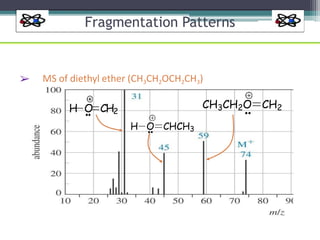
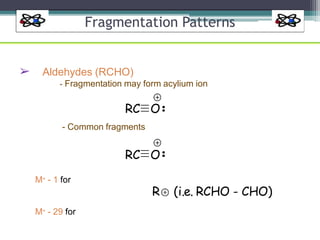
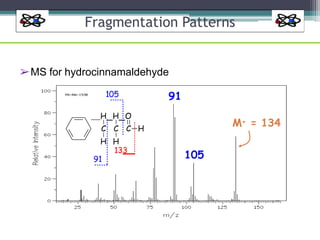
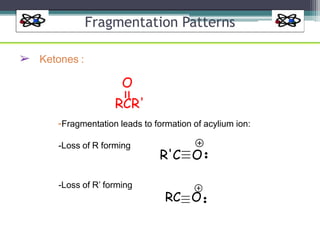
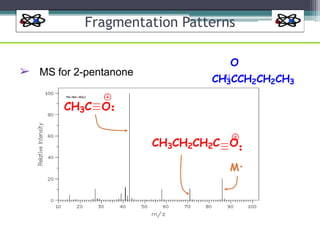
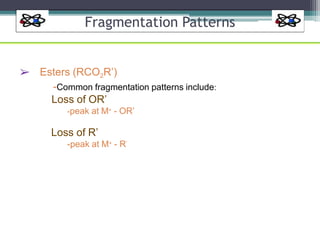
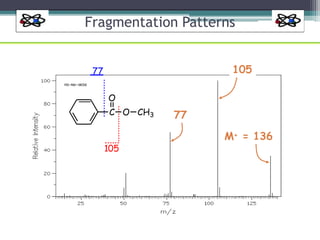
The document discusses isotopes, their effects on reaction rates, and fragmentation in mass spectrometry. It explains kinetic isotope effects, detailing primary and secondary effects related to bond formation and breaking, and outlines various fragmentation modes and rules for predicting ion peaks in mass spectrometry. Key principles regarding cation stability, bond cleavage, and resulting fragments in organic compounds are also presented.