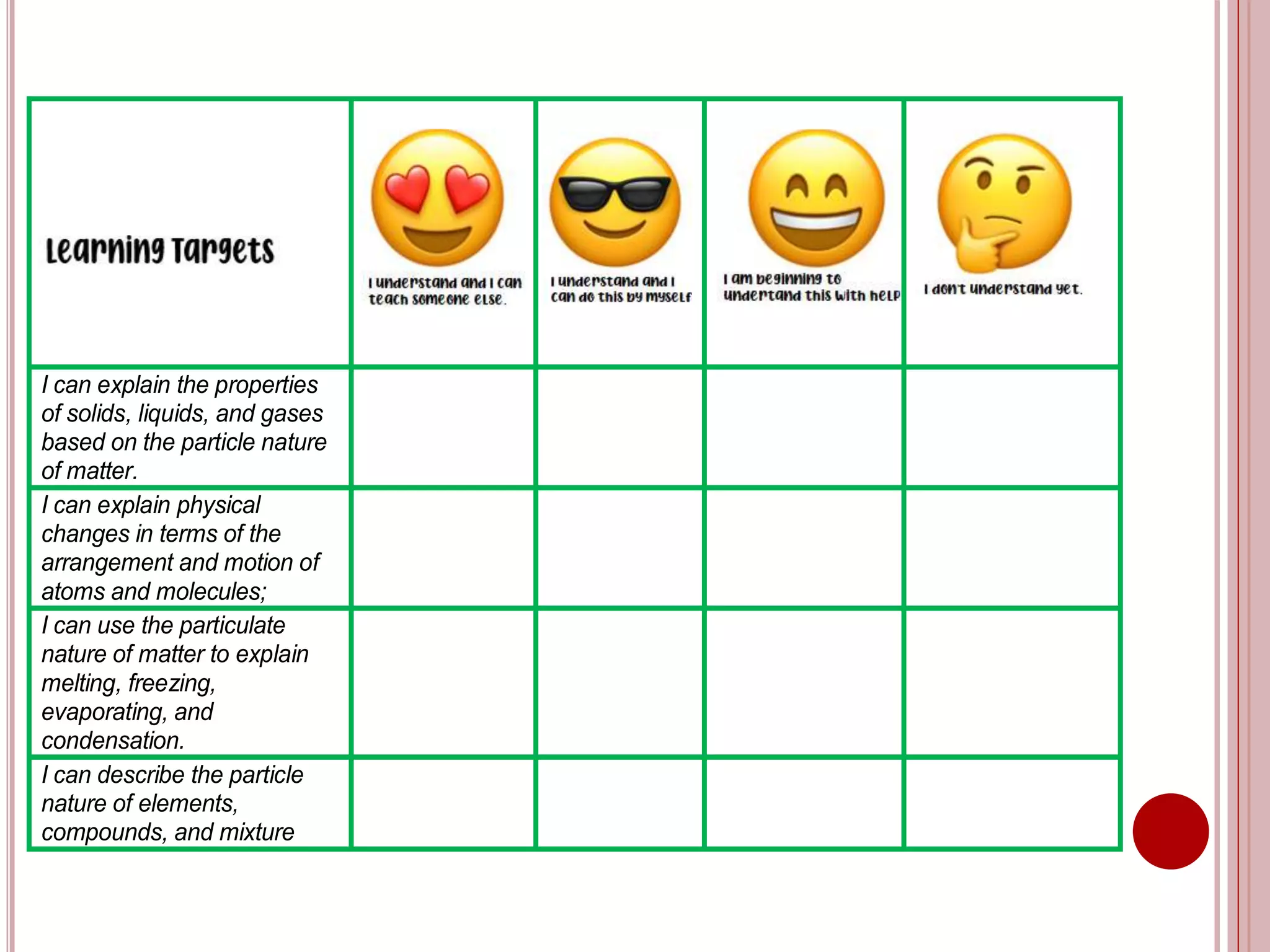
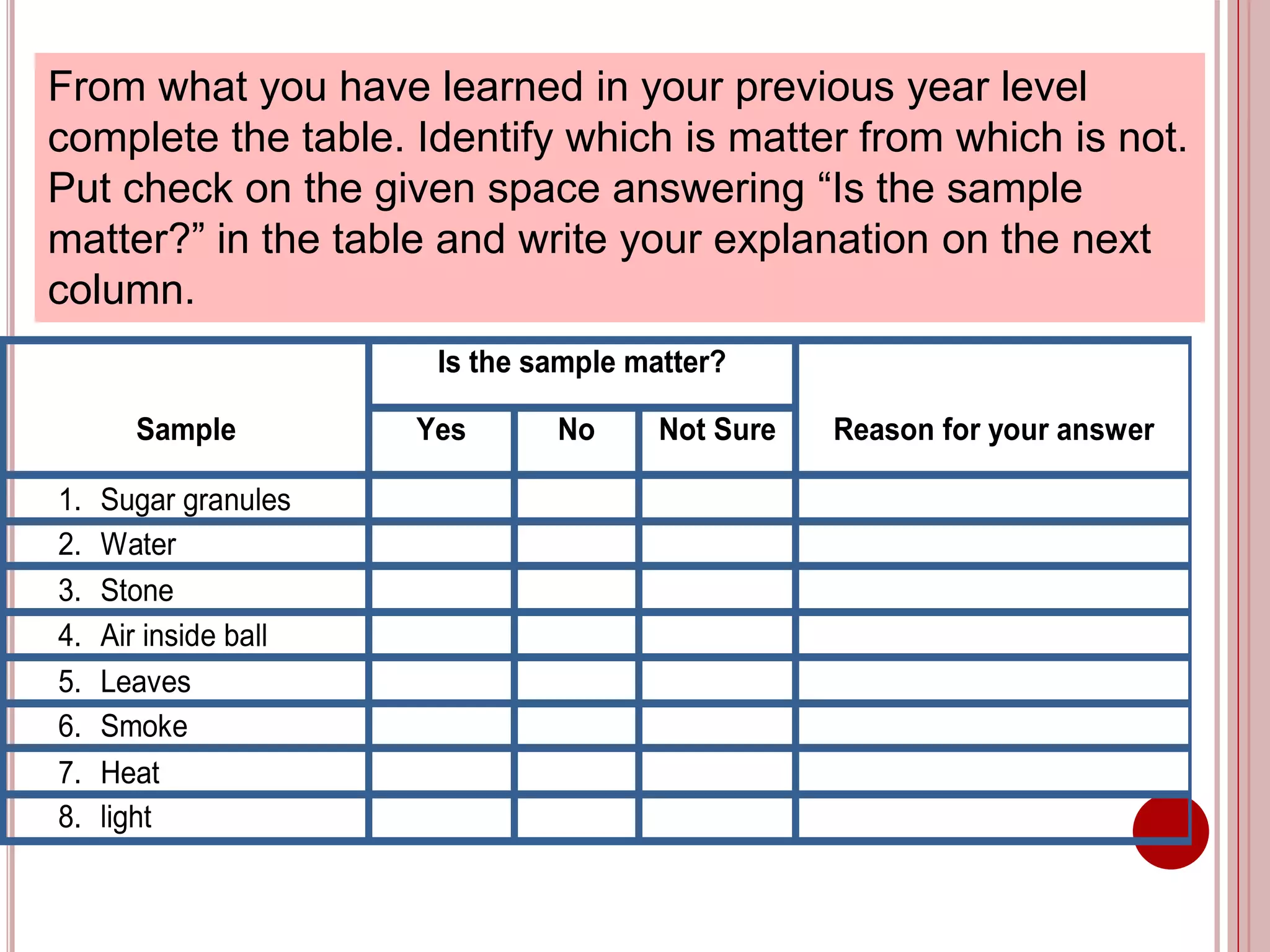
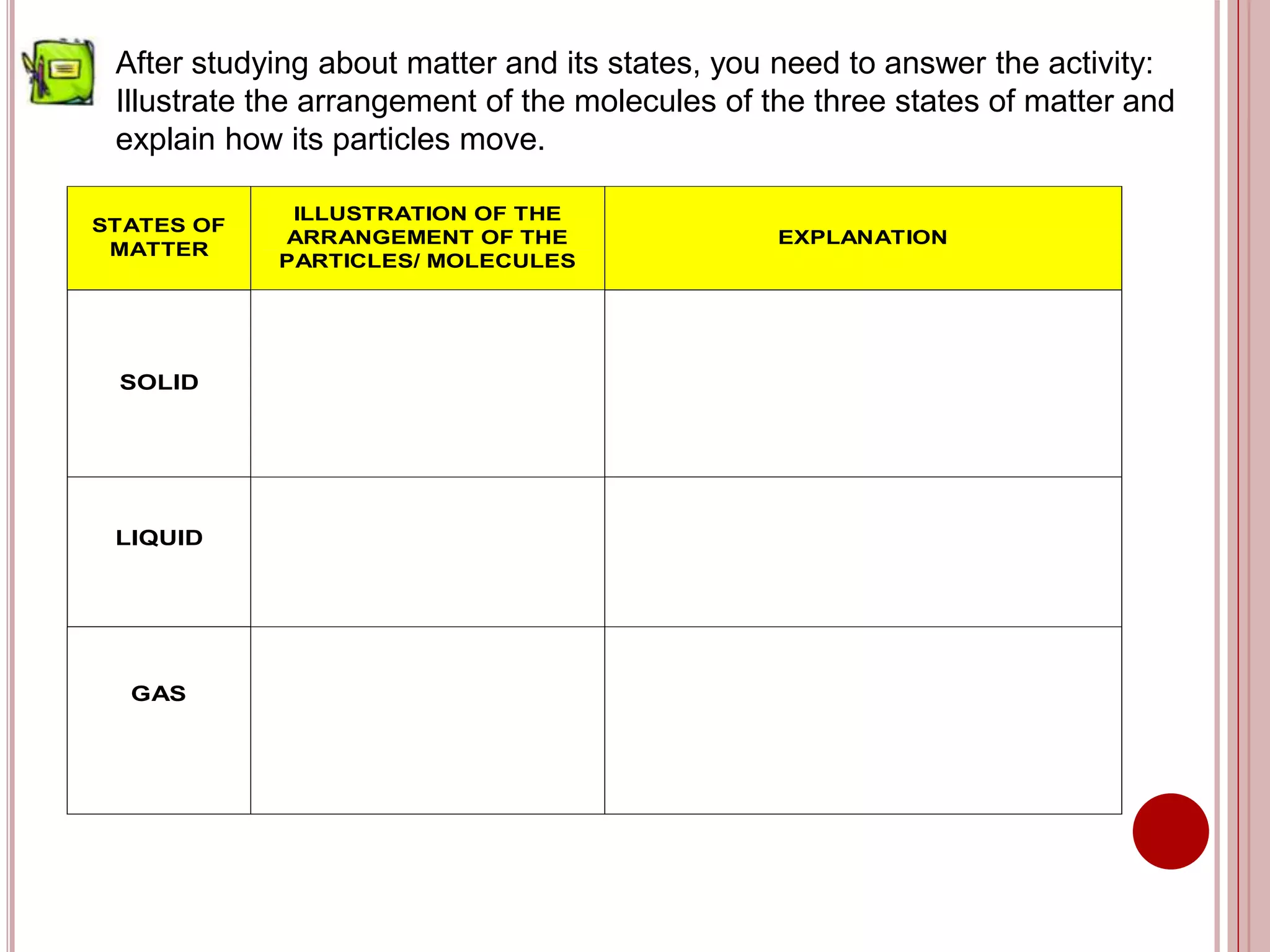
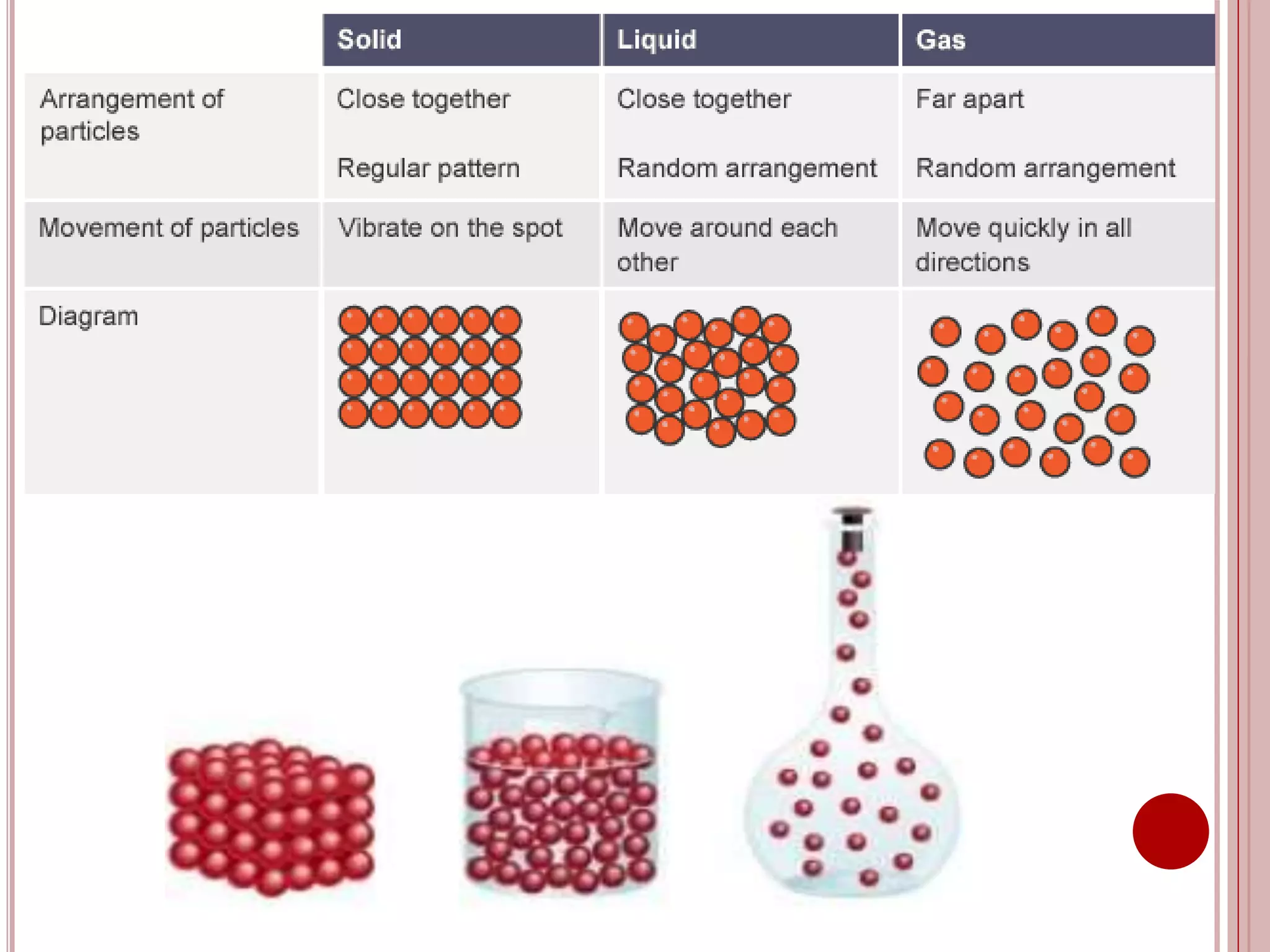
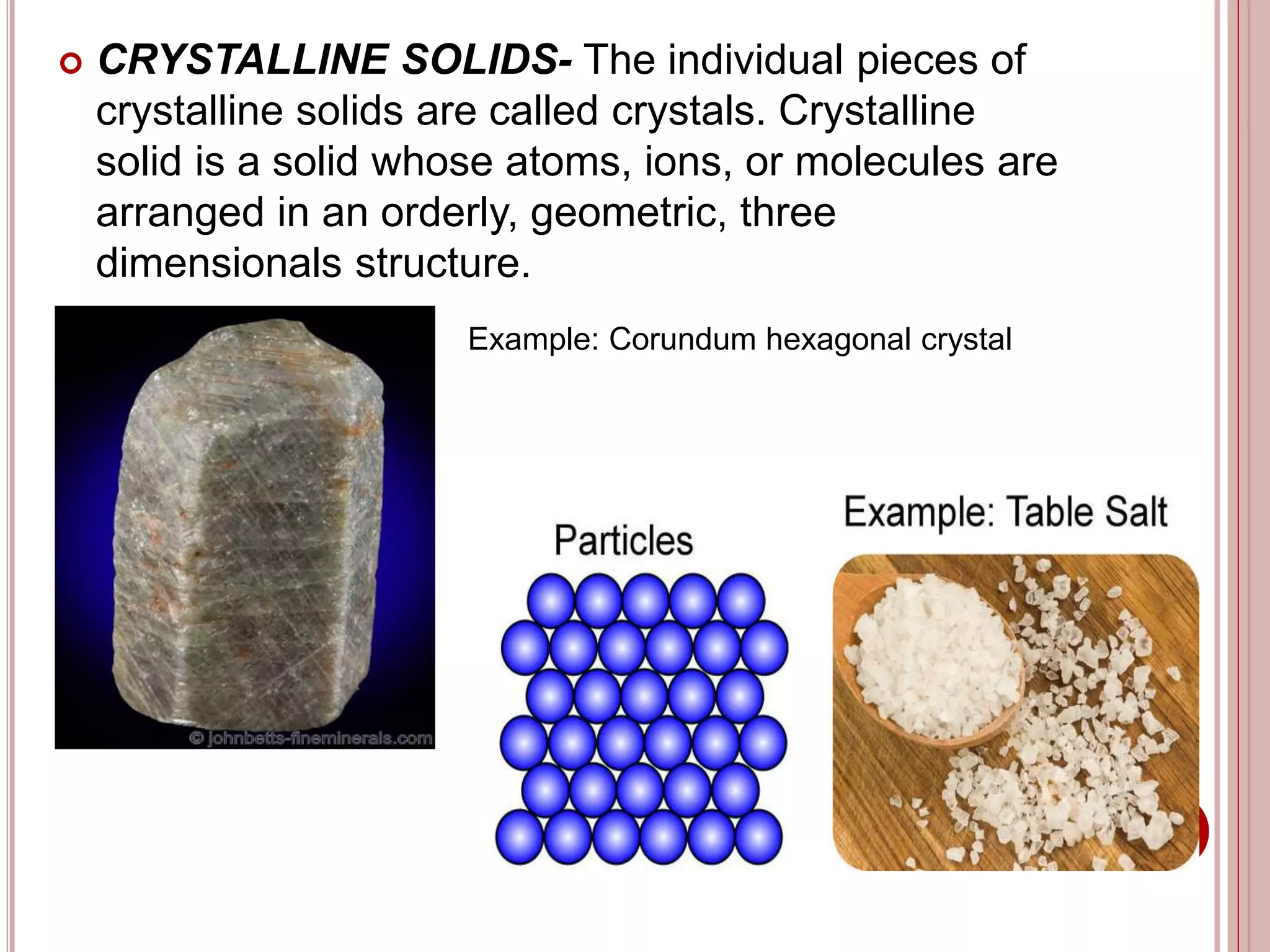
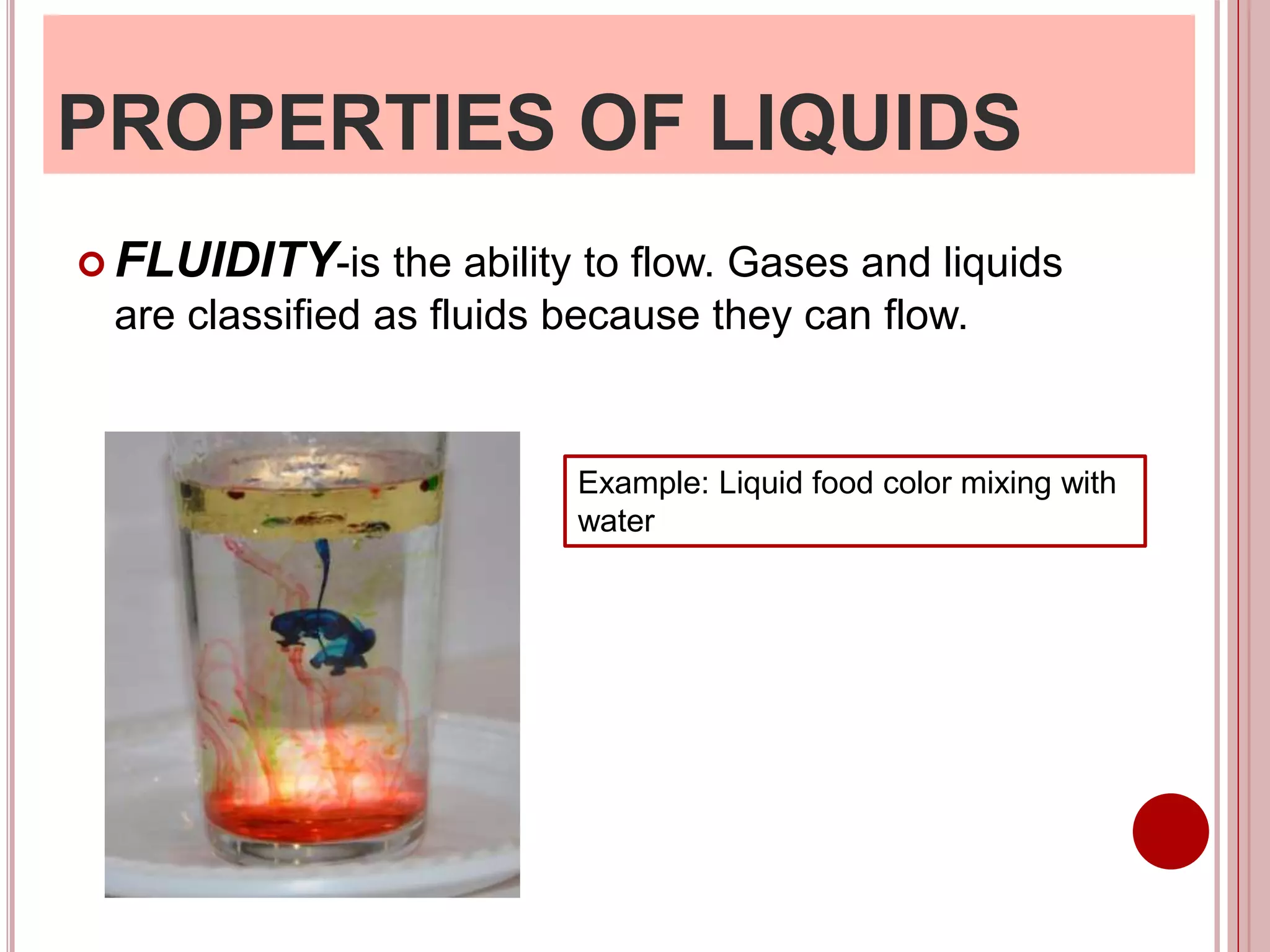
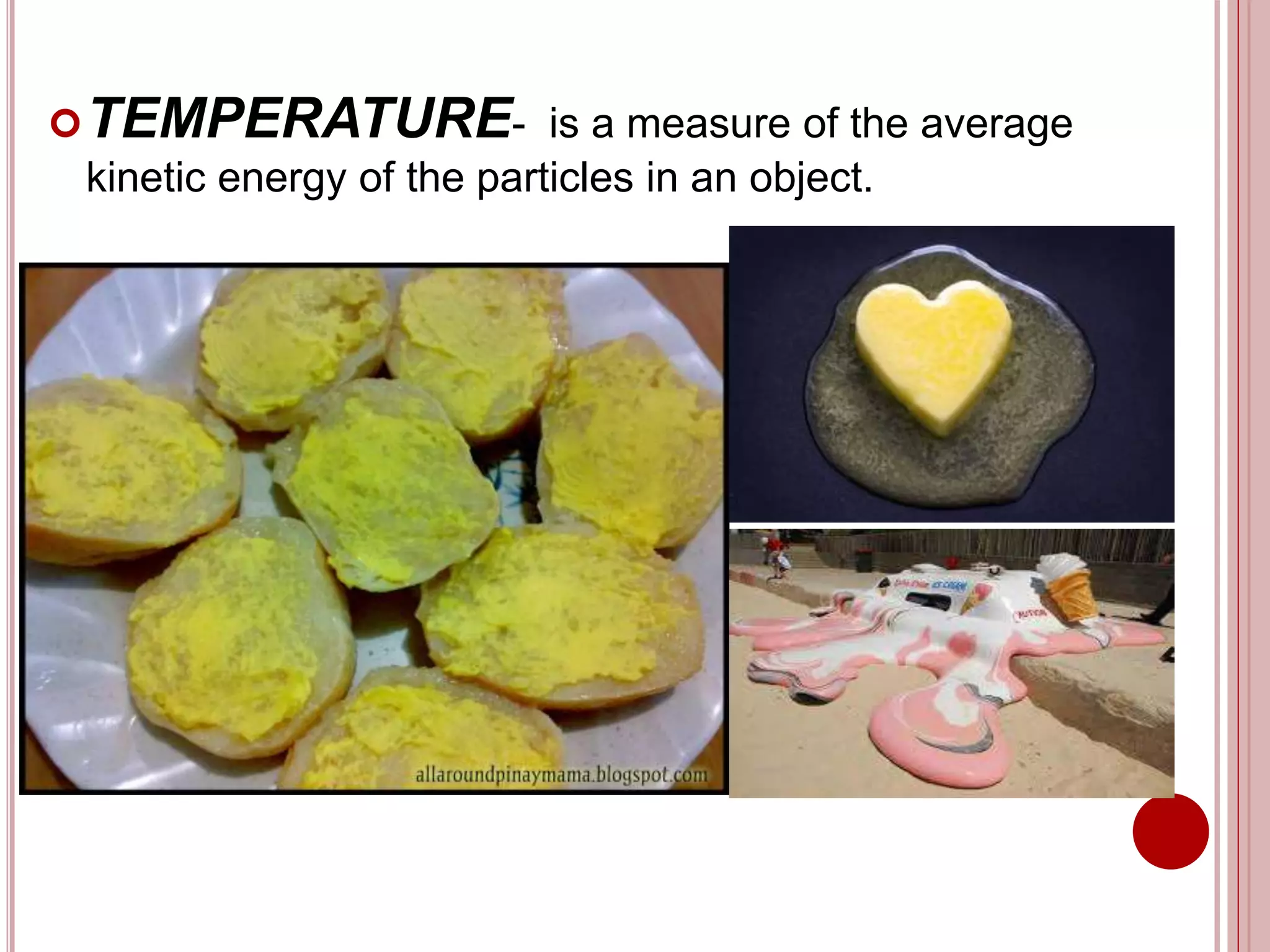
This document provides information about the particle nature of matter. It includes standards and objectives about demonstrating an understanding of the particle nature of matter and its properties. It discusses the key concepts of explaining the properties of solids, liquids, and gases based on how the particles are arranged and move. It also explains physical changes in these states in terms of particle arrangement and motion, and how this can be used to explain processes like melting, freezing, evaporating, and condensing.