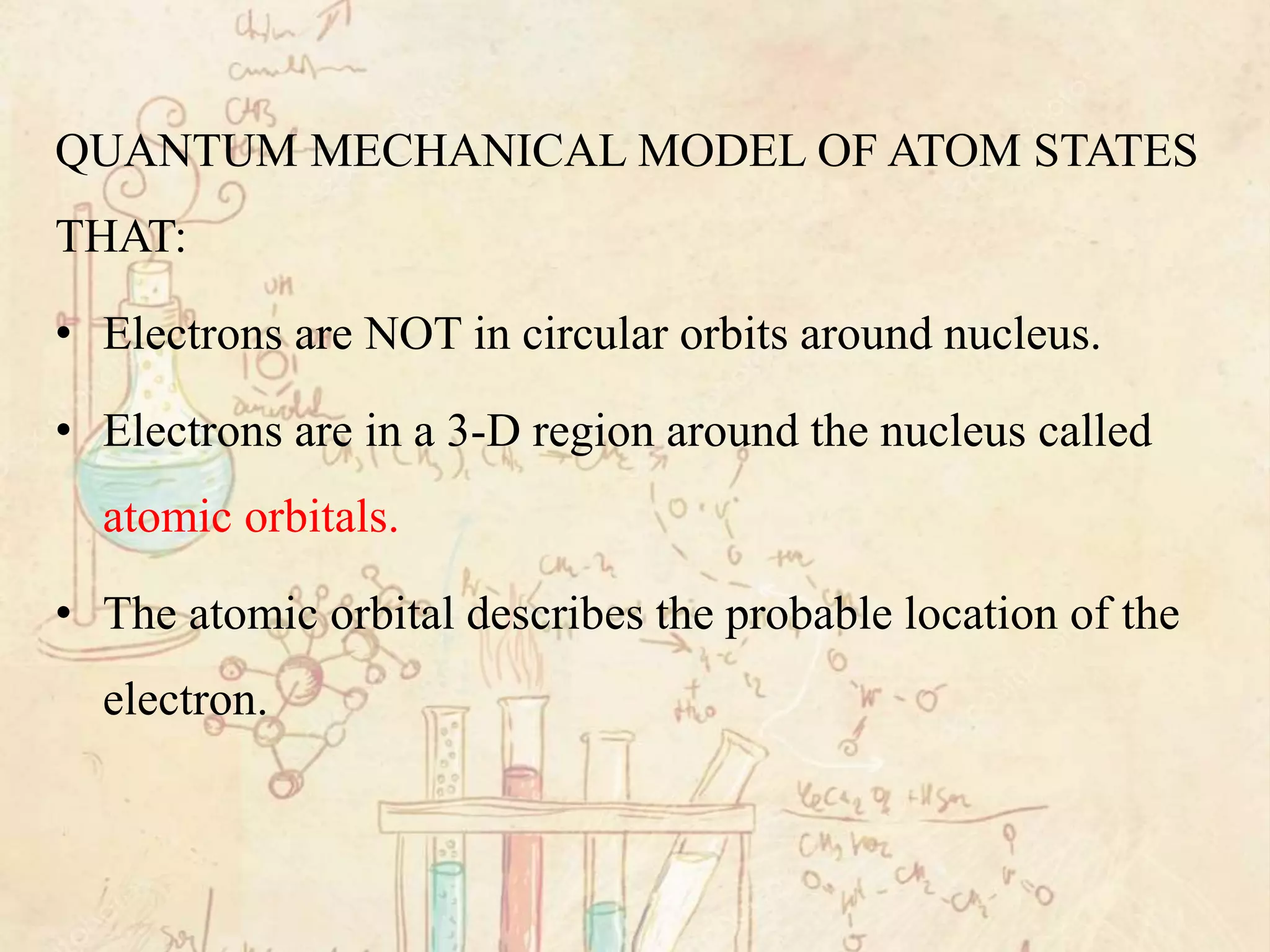
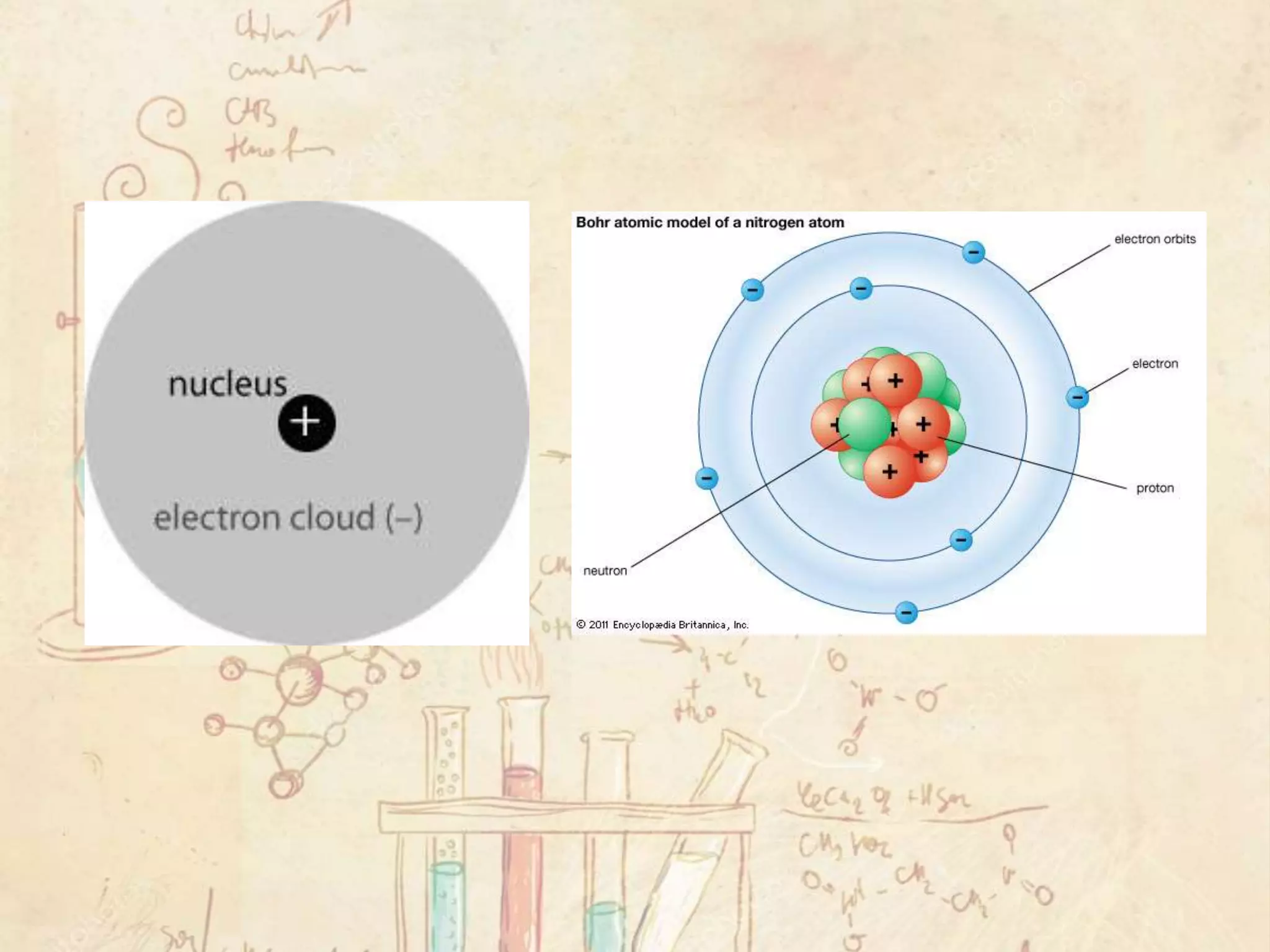
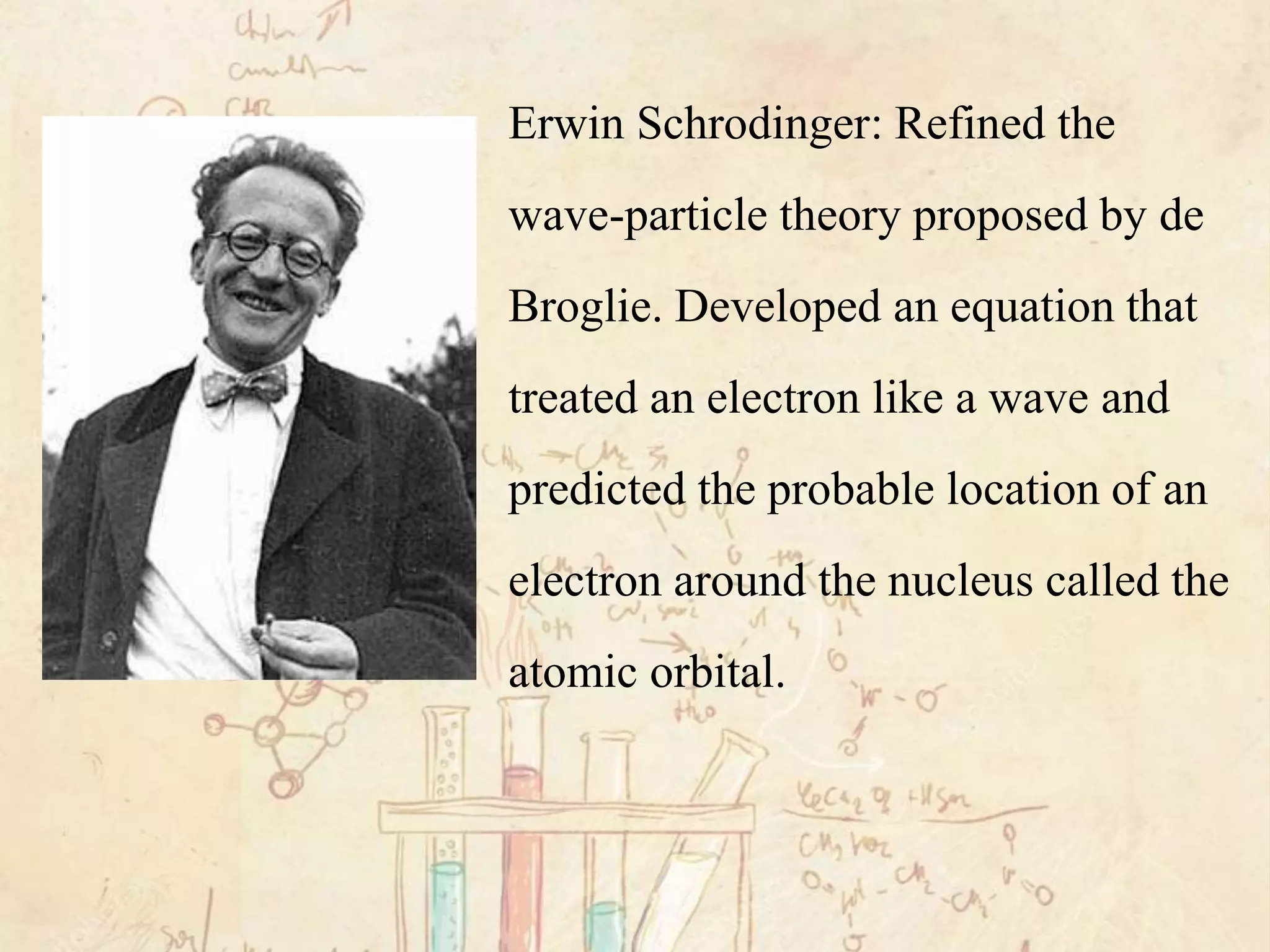
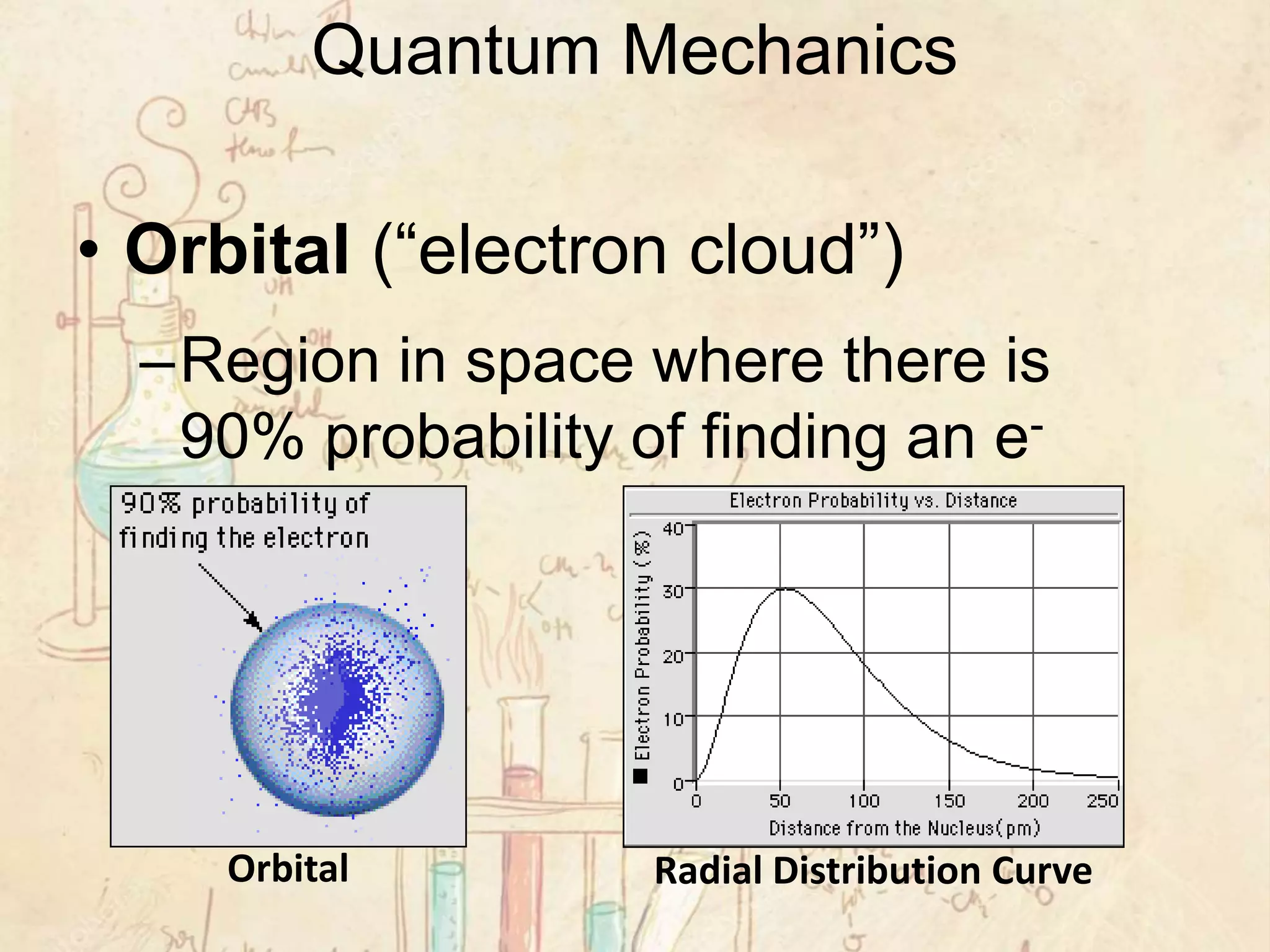
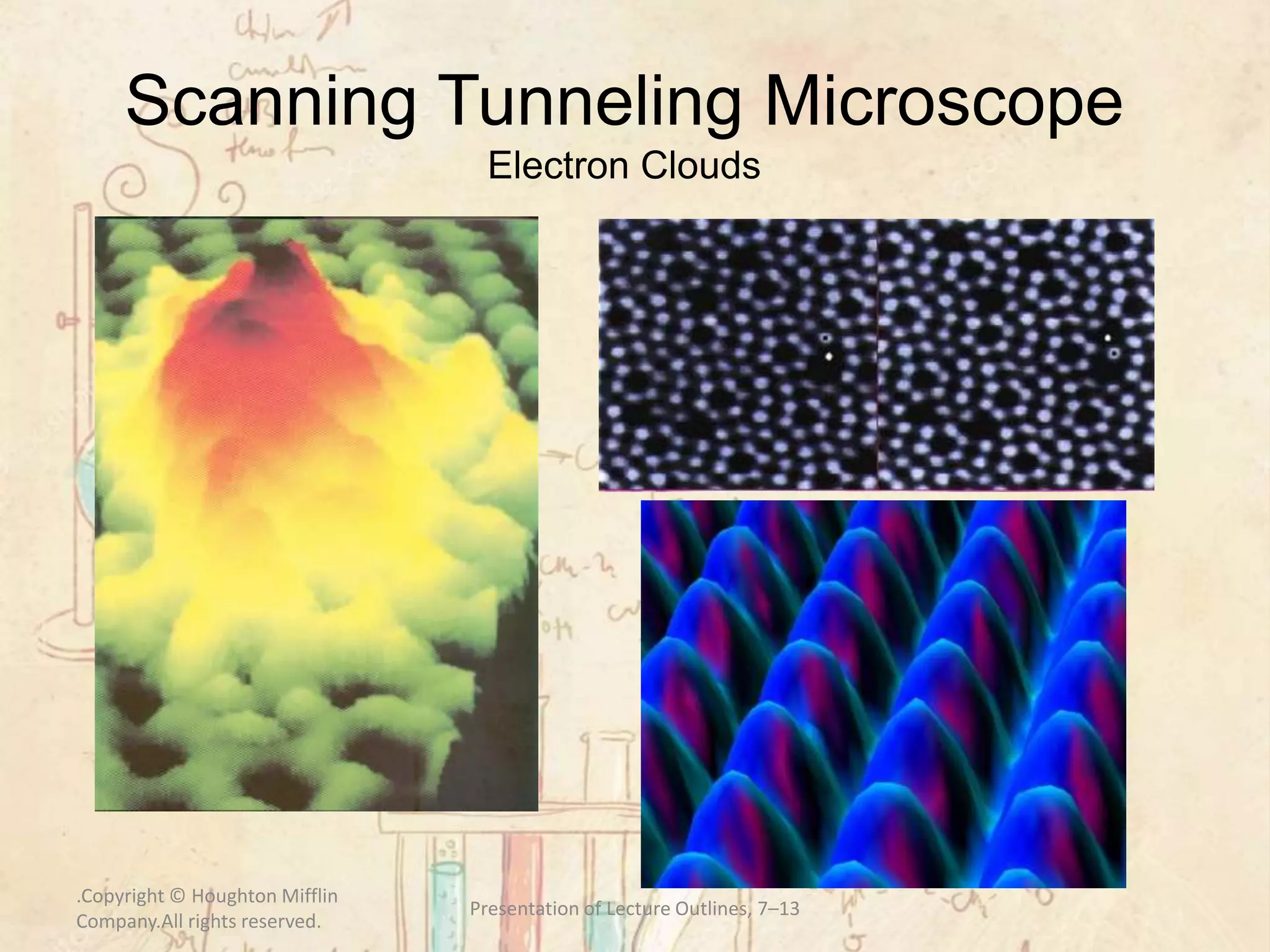
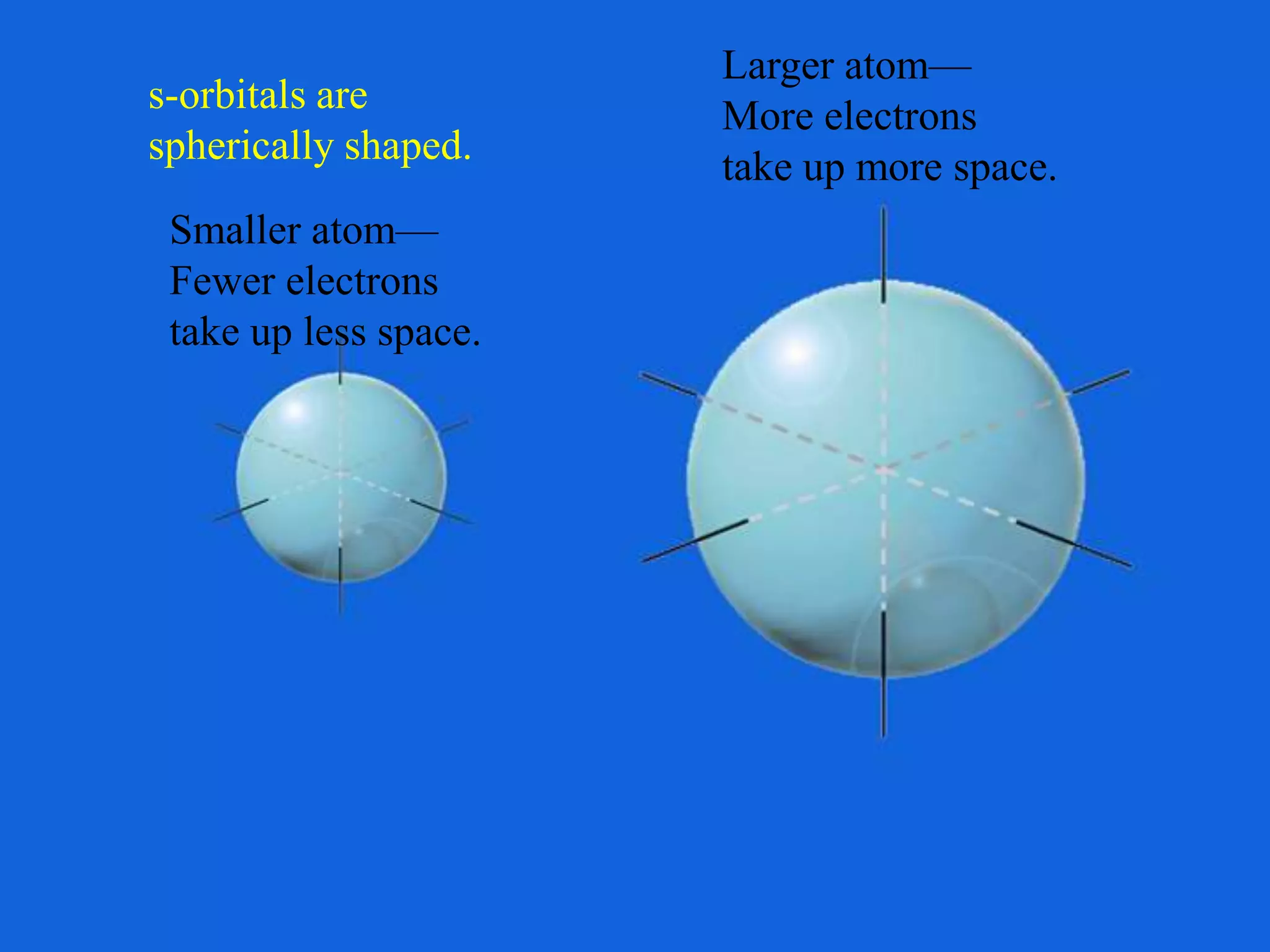
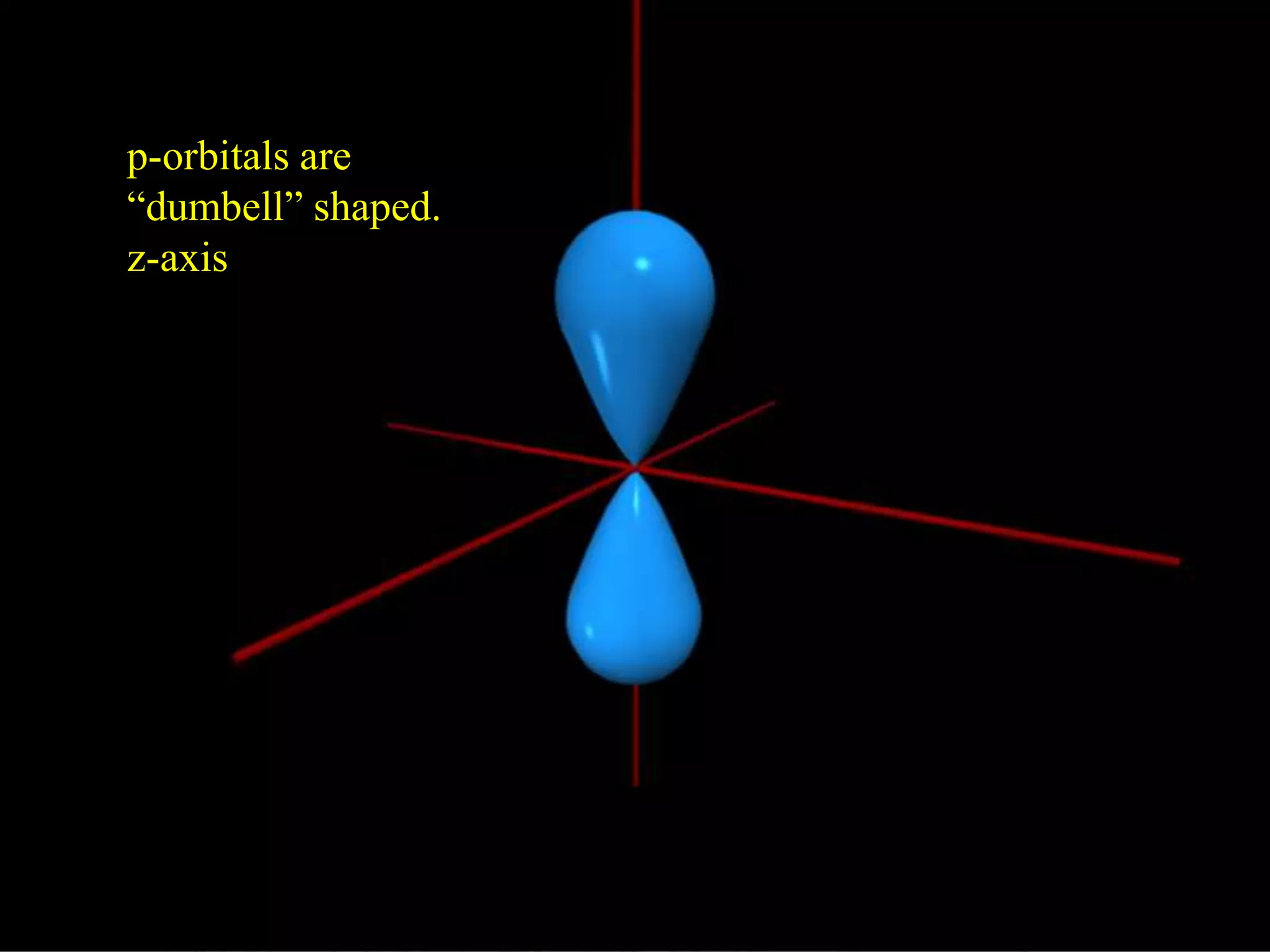
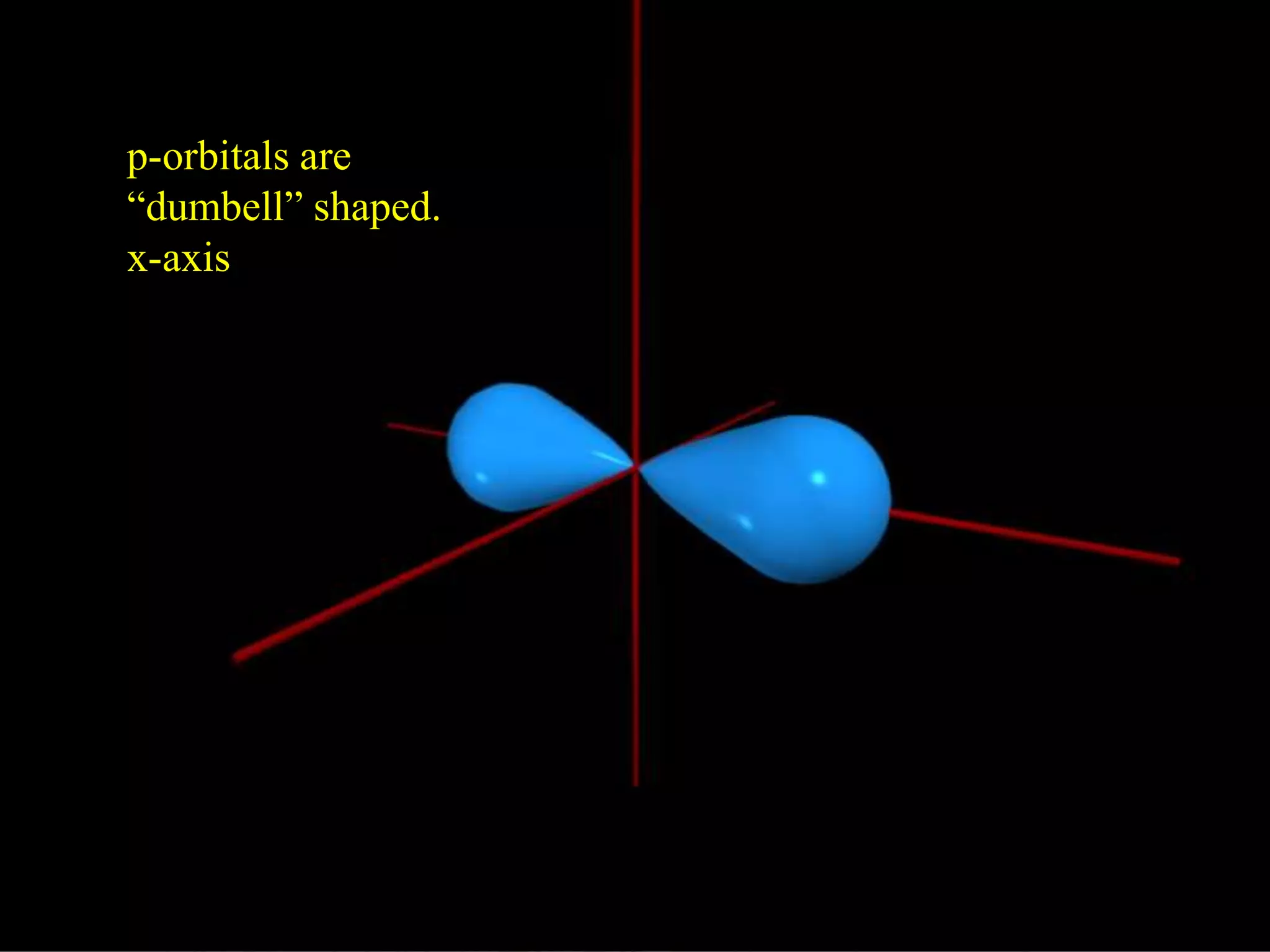
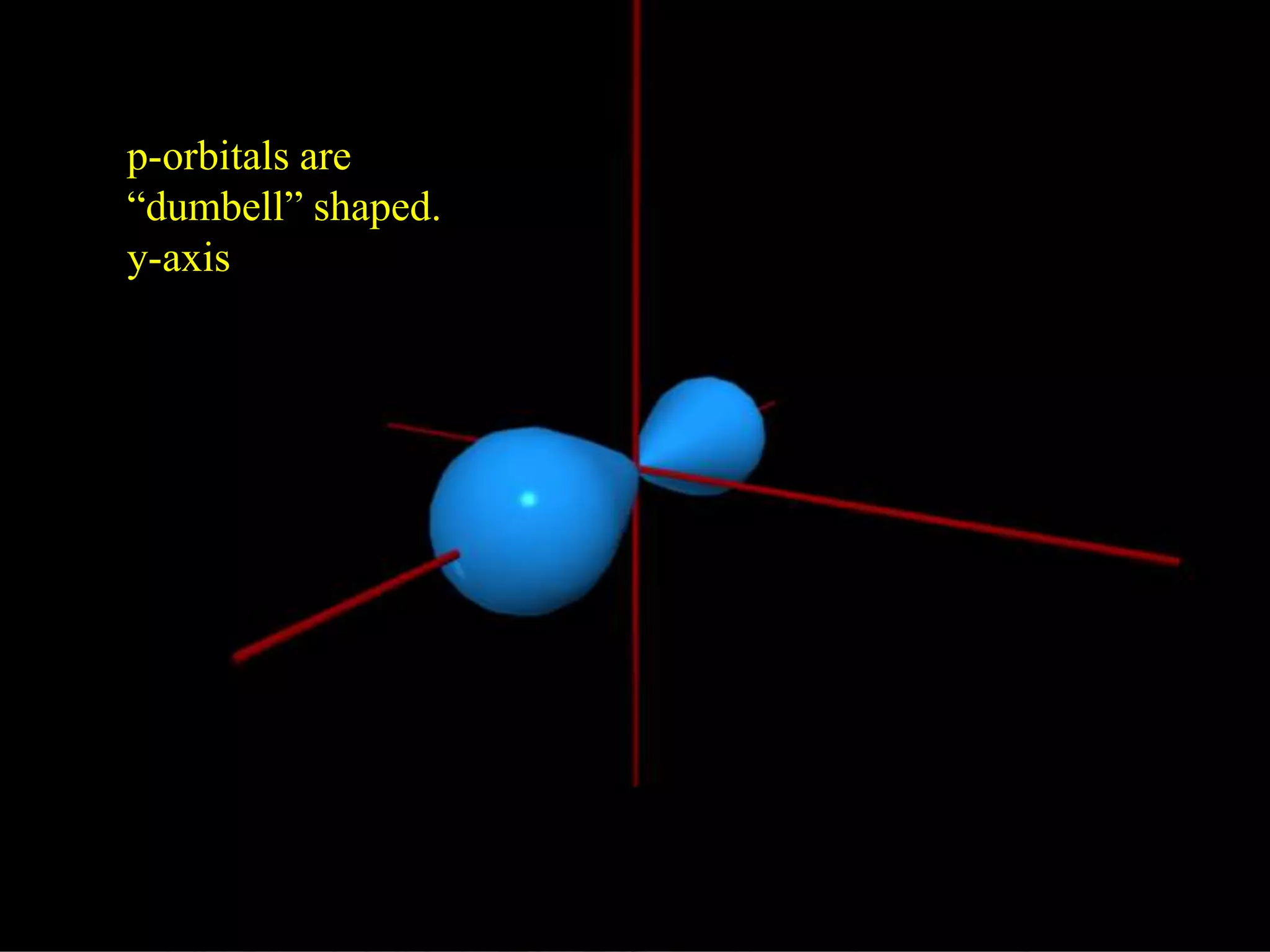
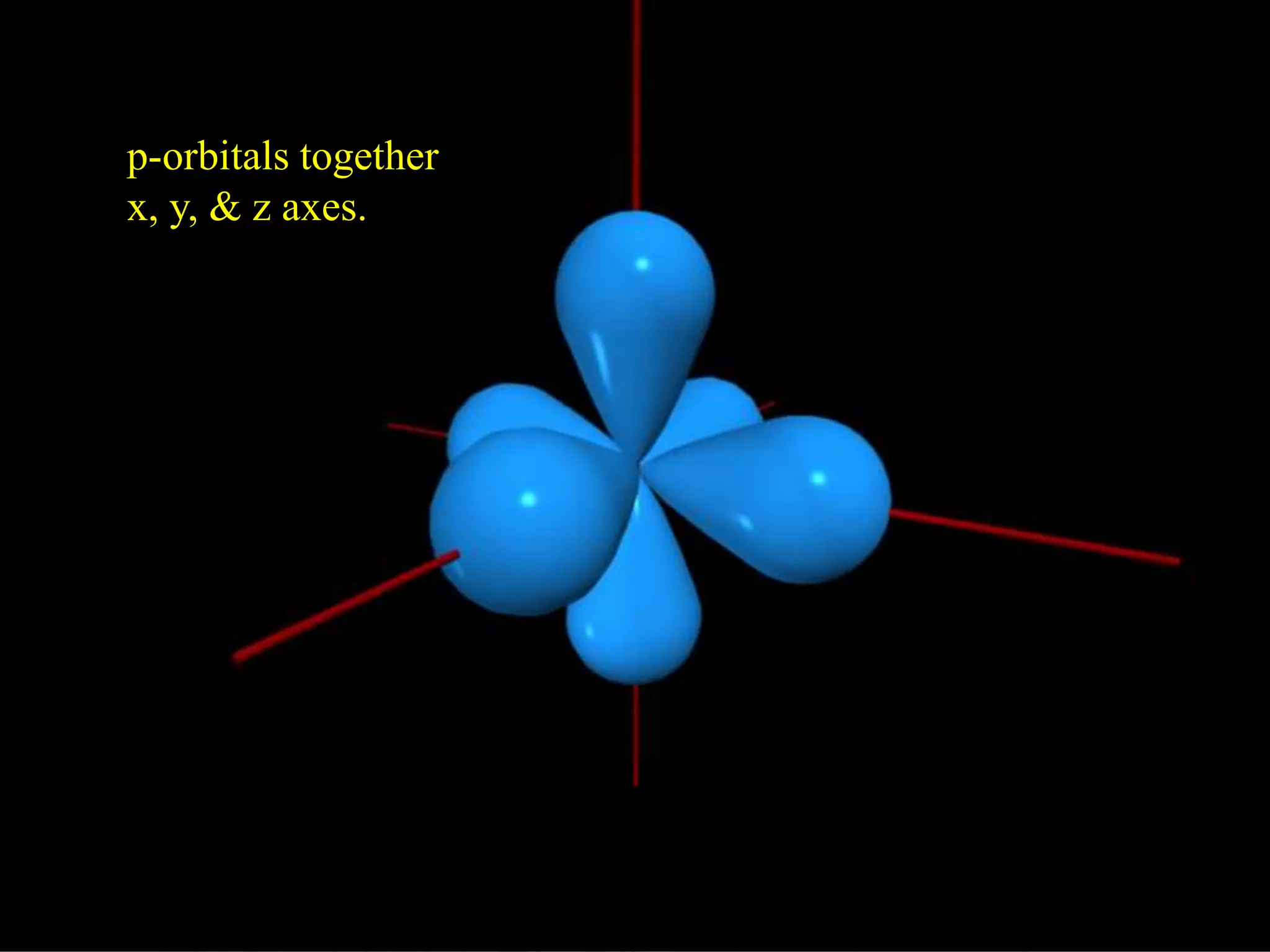
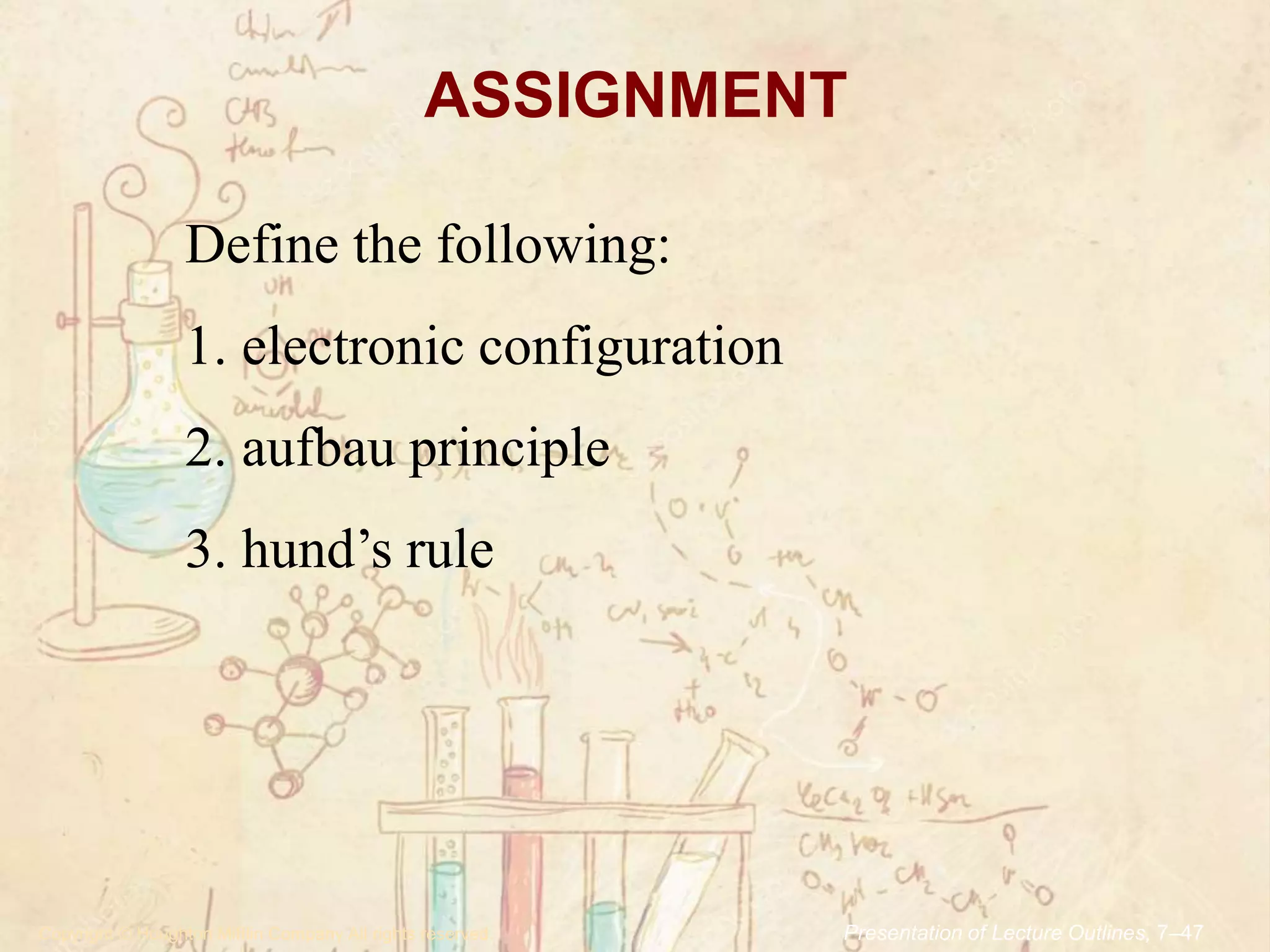
Here are the definitions:
1. Electronic configuration is the distribution of electrons of an atom or ion in atomic orbitals of the electron shells.
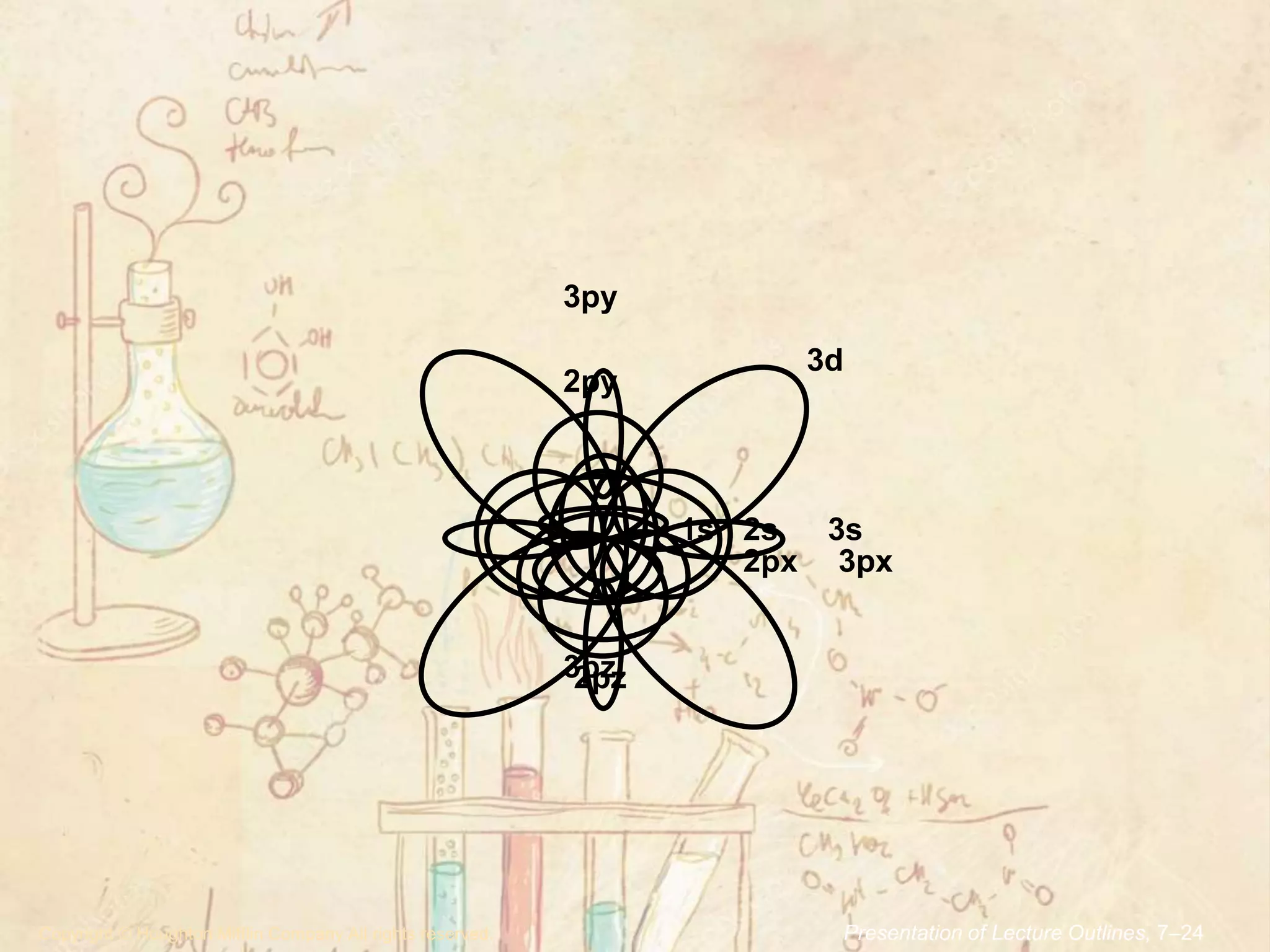
2. The aufbau principle states that electrons fill atomic orbitals of an atom in order of increasing energy level. Orbitals within a given energy level are filled first before the next energy level is begun.
3. Hund's rule states that when electrons are added to orbitals of the same energy in an atom or ion, they will occupy different orbitals singly, with their spins parallel, before any orbital is occupied by a second electron.