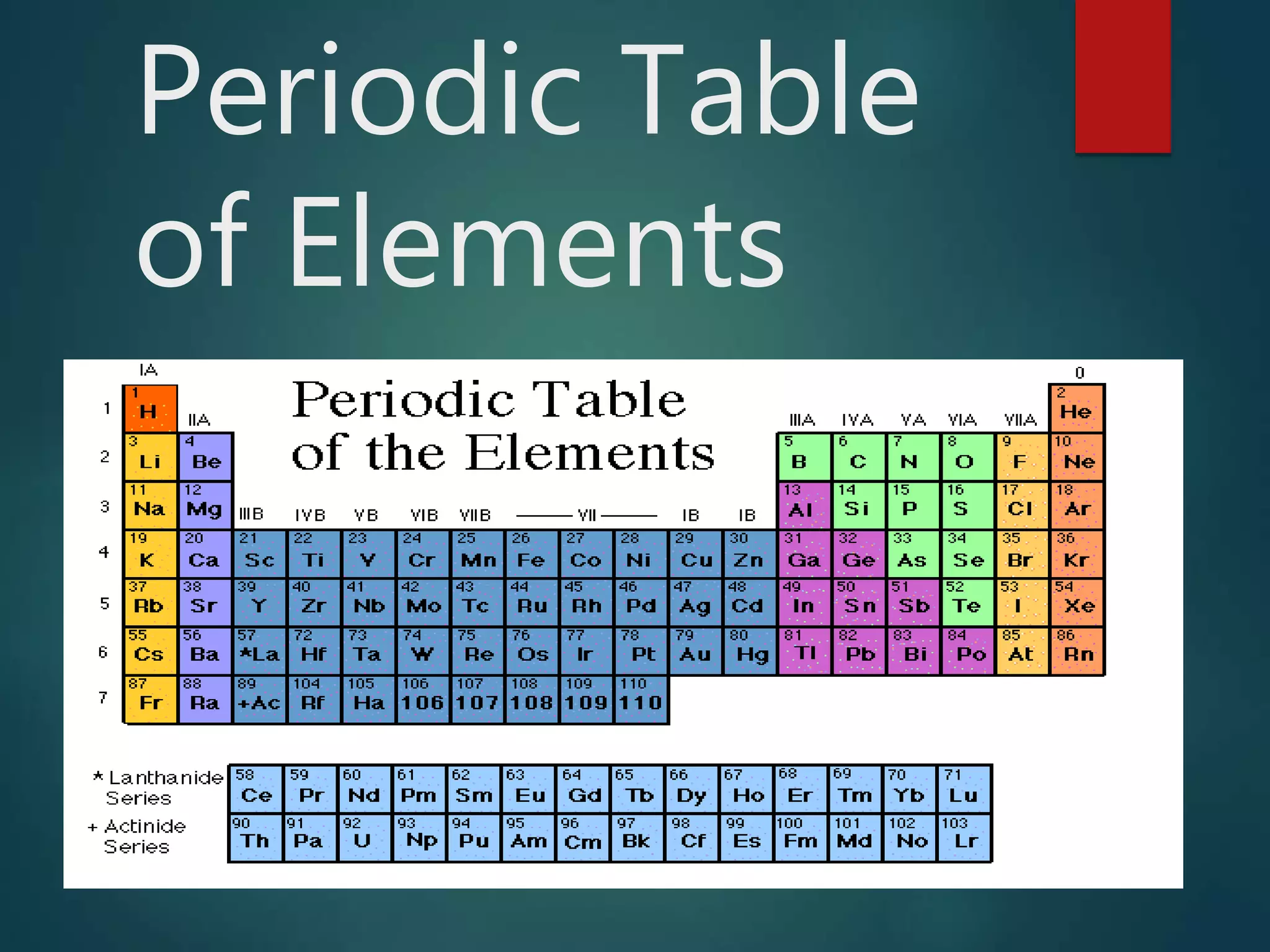
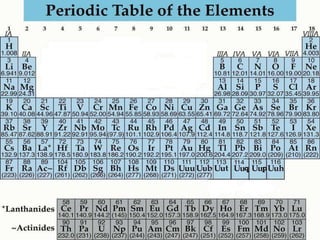
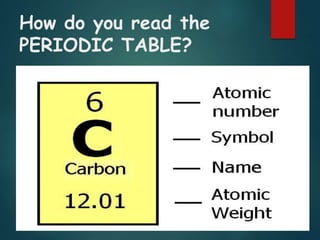
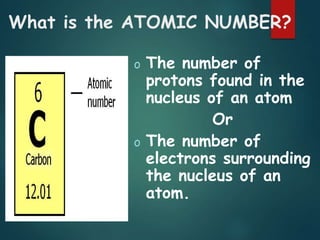
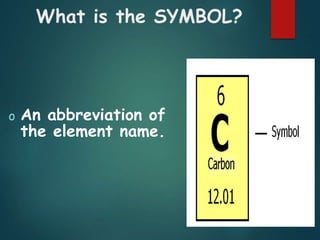
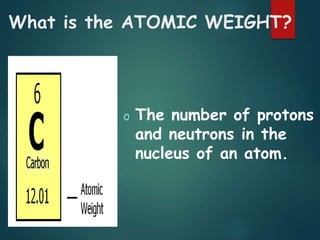
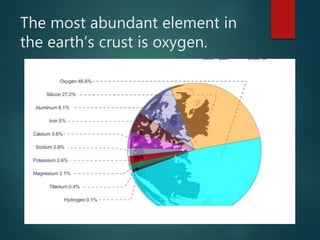
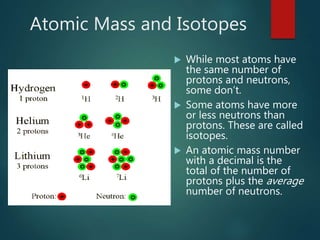
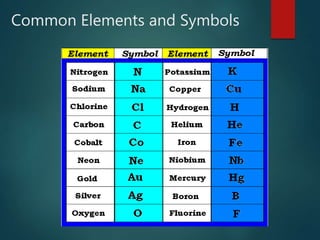
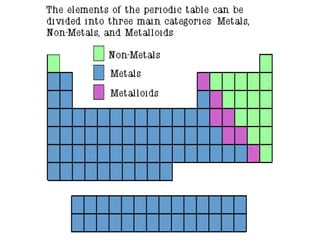
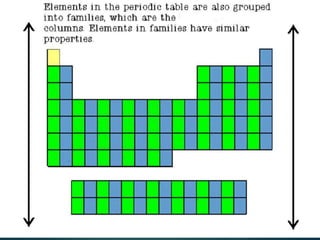
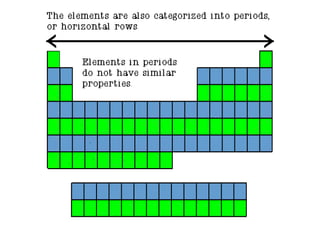
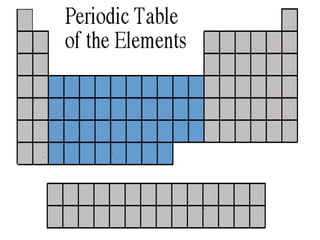
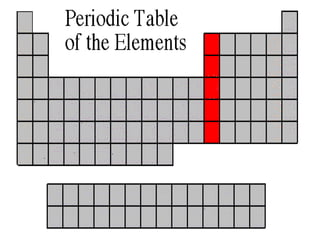
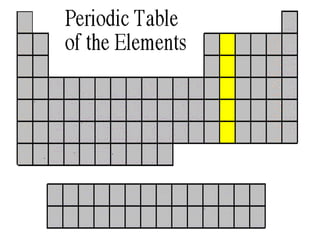
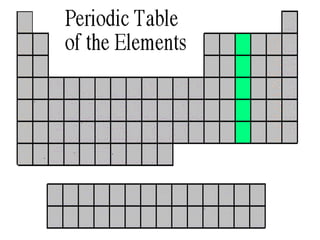
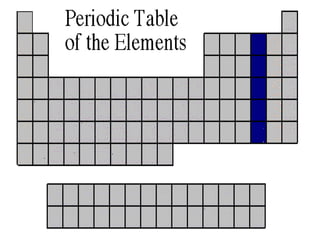
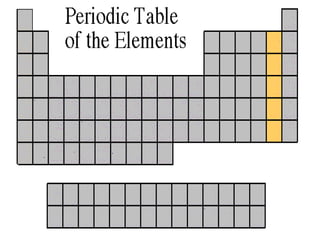
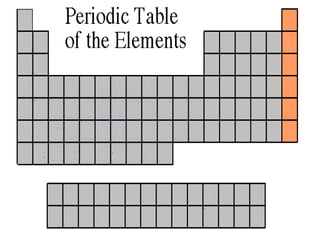
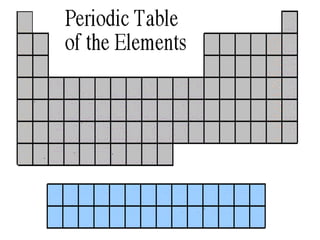
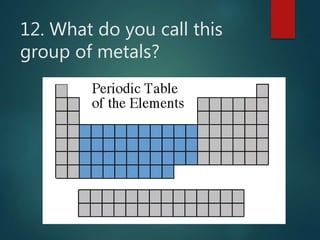
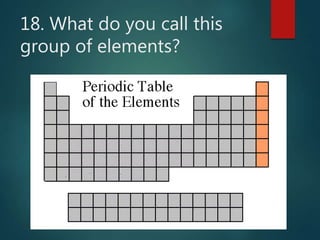
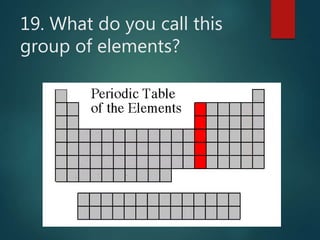
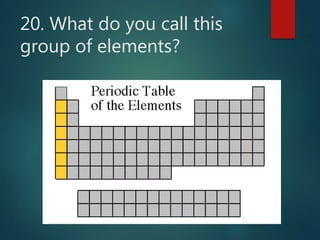
The document discusses the periodic table of elements created by Dmitri Mendeleev in 1869. It organizes all known elements based on their atomic structure and chemical properties. Key terms defined include atomic number, symbol, atomic weight, protons, electrons, neutrons, metals, non-metals, metalloids, periods, families, and properties of common element groups such as alkali metals, halogens and noble gases. The periodic table arranges elements horizontally by atomic number and vertically into families with similar traits.