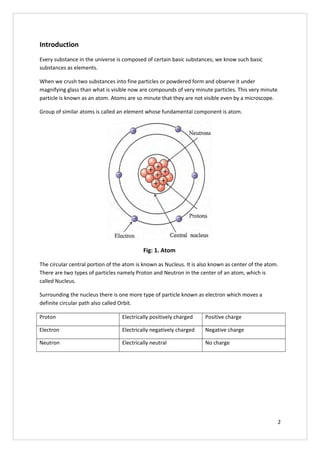
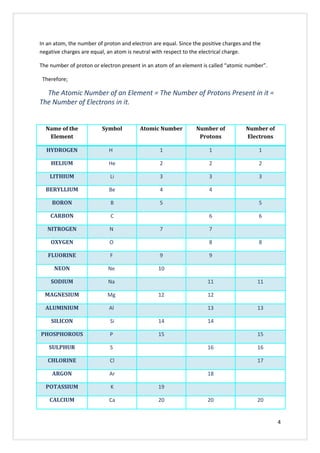
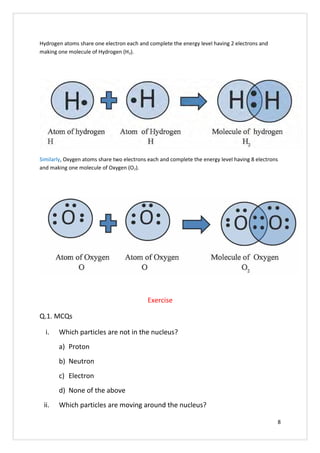
This document discusses the molecular structure of atoms. It begins by explaining that all substances are composed of elements, which are the basic substances, and that elements are made up of very small particles called atoms. It then describes the basic structure of an atom, including that atoms have a small, positively charged nucleus surrounded by negatively charged electrons. The number of protons determines the element and equals the number of electrons. Atoms can gain, lose, or share electrons to attain stable electron configurations, forming ions or molecules.