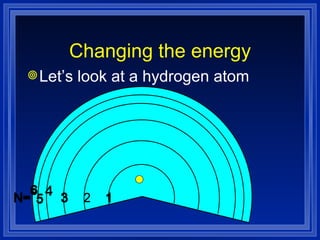
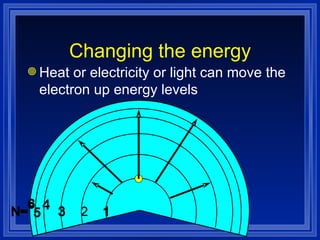
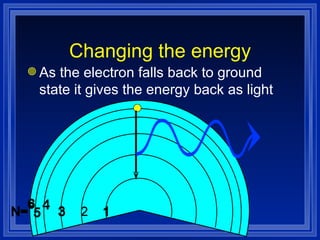
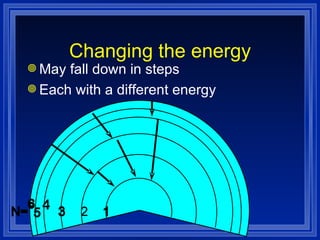
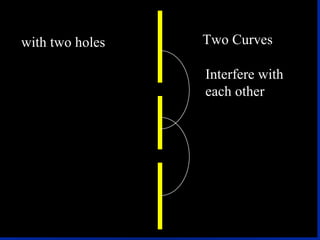
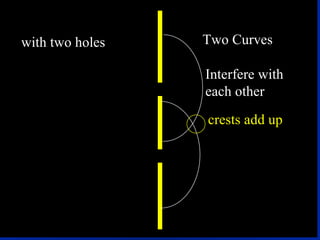
1) The document discusses the quantum mechanical model of the atom, including atomic orbitals and electron configurations.
2) It describes how electrons can occupy different atomic orbitals based on their energy levels and how the electron configuration notation is used to show the filling of these orbitals.
3) It also mentions exceptions to the expected electron configuration filling order that result in more stable half-filled or filled orbitals.