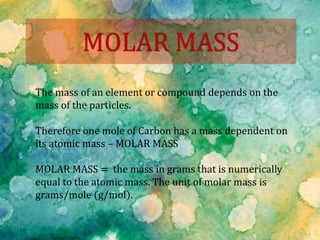
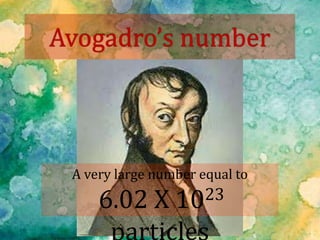The document provides instructions for conducting an experiment to measure the mass of paper clips using a balance and compute the number of paper clips in a handful based on the average mass. It asks students to record their results in a table and compare the computed number of paper clips to the actual counted number, explaining any differences. It then provides background information on moles as a unit of measurement in chemistry, defining it as 6.02 x 1023 particles and providing examples of converting between numbers of particles and moles for different substances using molar mass.