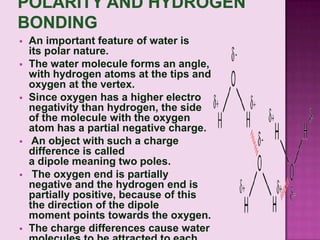
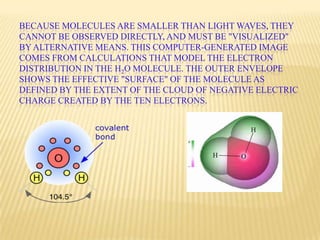
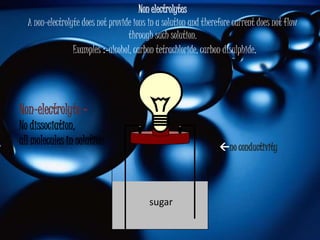
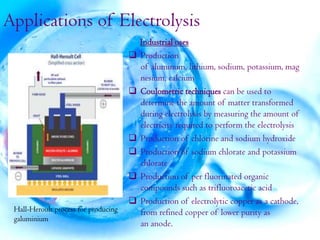
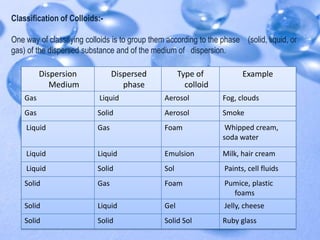
The document discusses the molecular structure of water. It explains that a water molecule is formed by two hydrogen atoms and one oxygen atom bonded together at an angle of 104.5 degrees. The polarity of the water molecule arises from oxygen's higher electronegativity which gives it a partial negative charge and the hydrogen atoms a partial positive charge. This polar nature is an important feature of water and causes it to have unique properties.