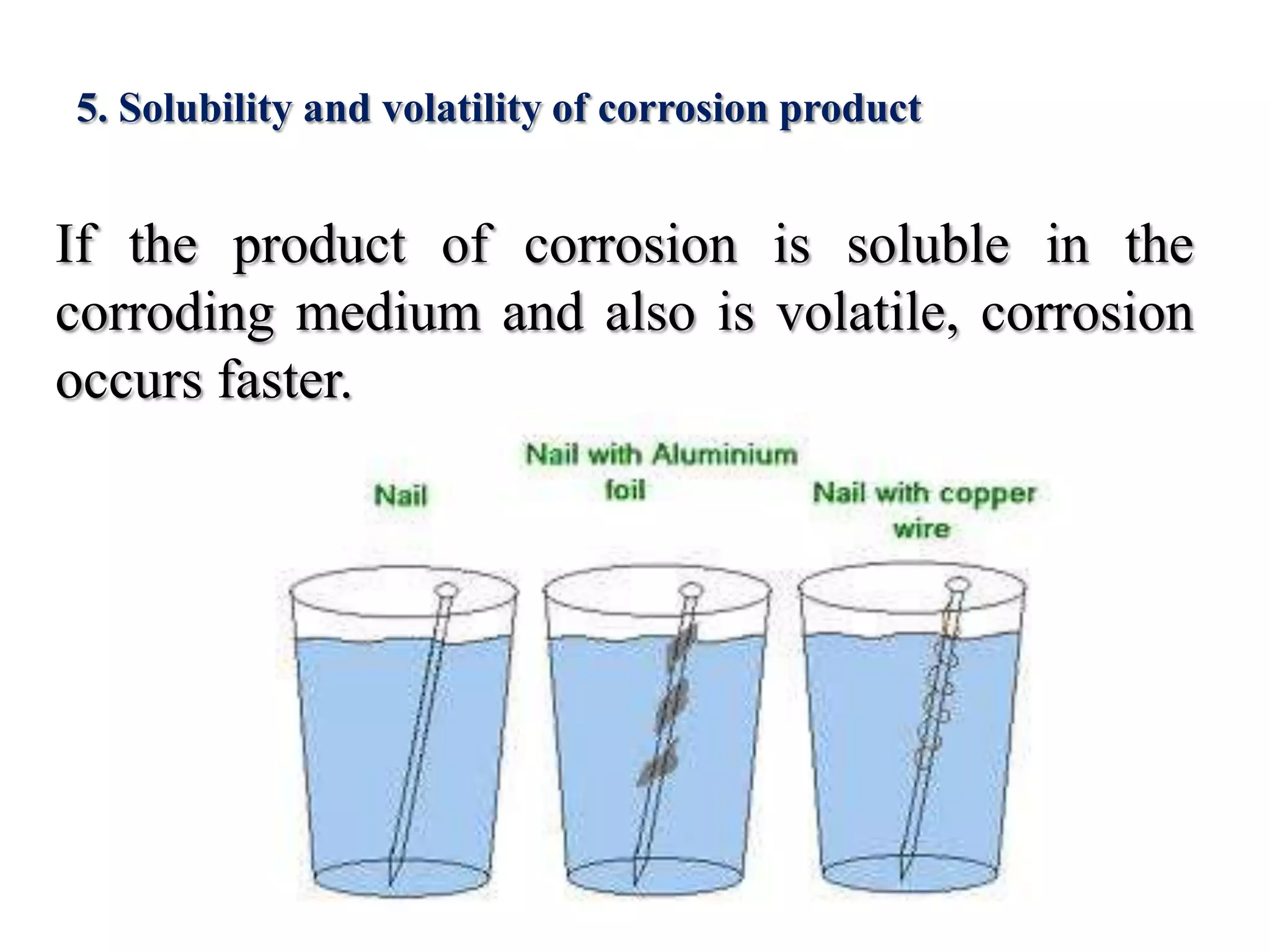
This document discusses corrosion and how it affects metals. It defines corrosion as the slow process of decay of metals due to chemical or electrochemical reaction with their environment. This causes the formation of compounds like oxides on the metal surface. Corrosion occurs through both dry chemical reactions and wet electrochemical reactions that set up galvanic cells. The rate of corrosion is affected by factors like the metal's position in the galvanic series, purity, environment, and characteristics of the corrosion products formed. Common types of corrosion discussed are rusting of iron and methods to prevent or reduce corrosion like coatings, cathodic protection, and modifying the environment.