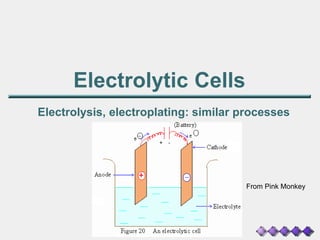
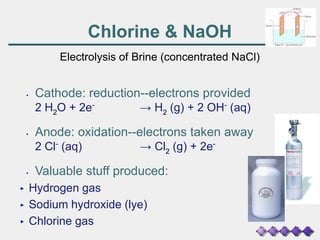
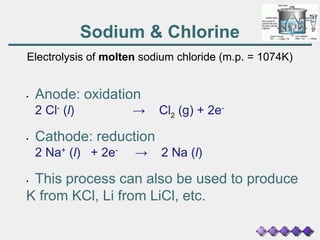
The document discusses the principles of electrolysis and electroplating, particularly focusing on the operation of electrolytic cells, such as lead-acid batteries and various electrolysis reactions. It outlines how batteries supply energy through the movement of electrons and highlights the production of valuable substances like hydrogen, chlorine, and sodium hydroxide through brine electrolysis. Additionally, it covers the stoichiometry of electrolysis to predict the amount of substance produced at electrodes based on current and time.