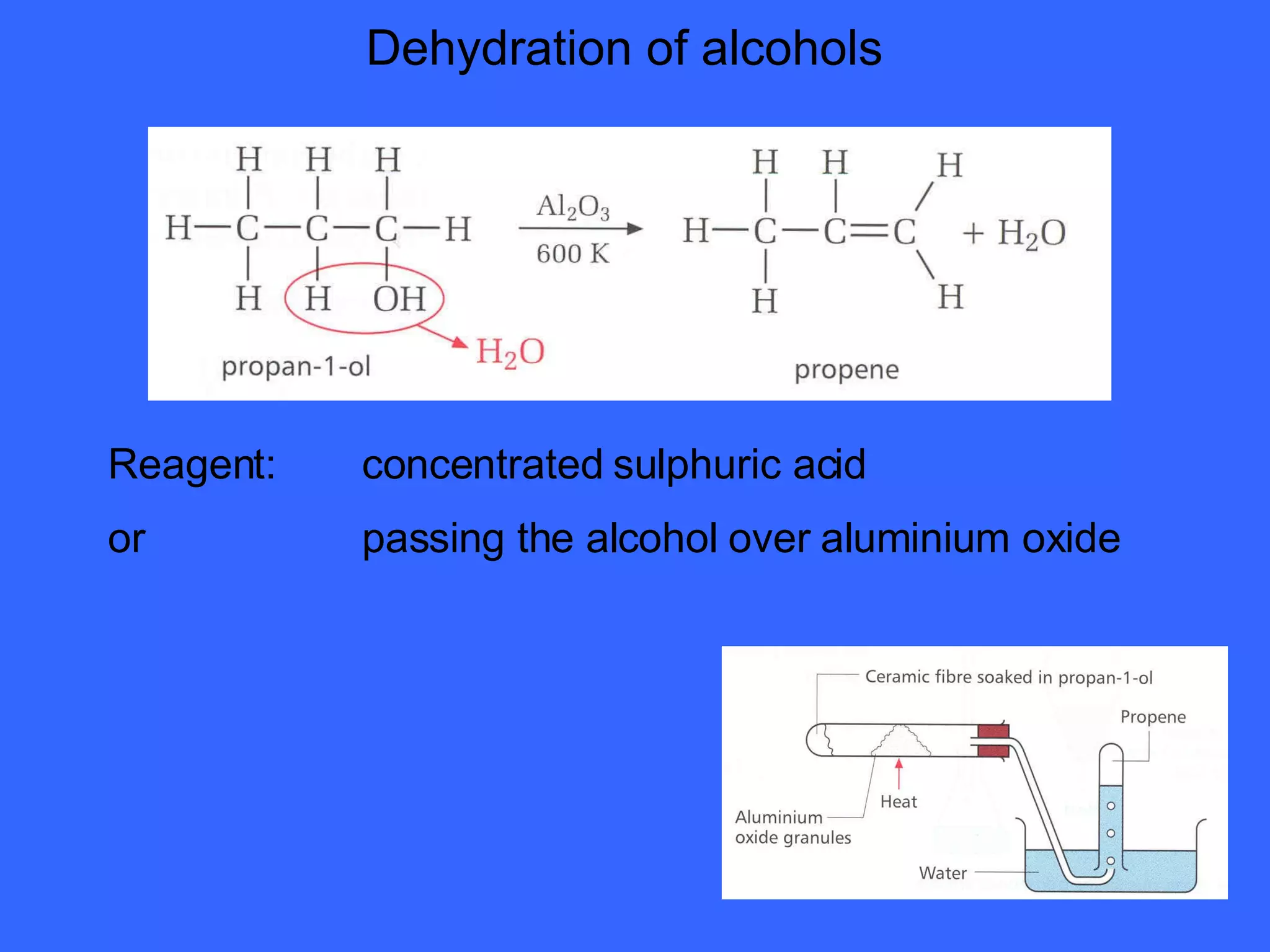
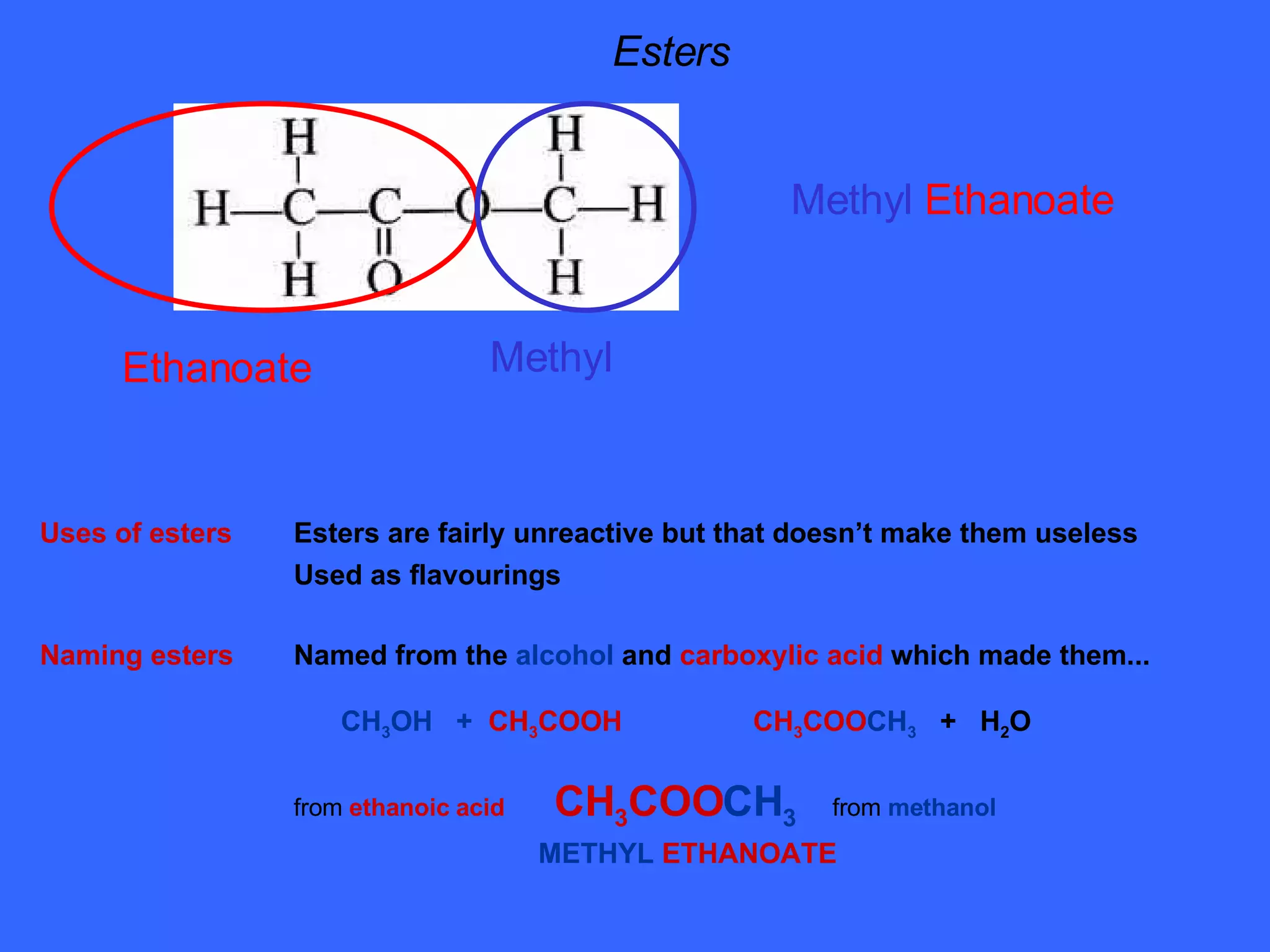
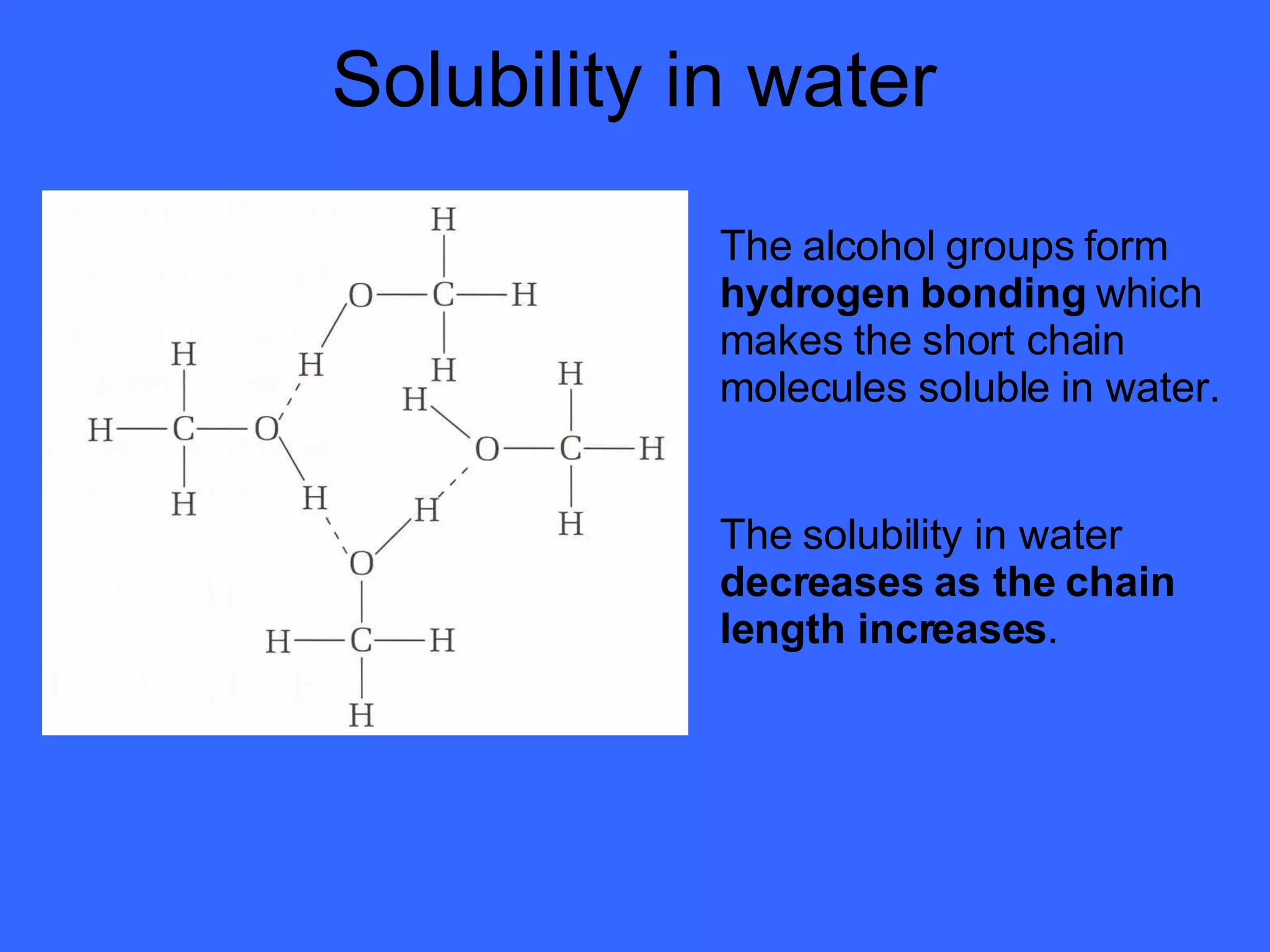
The document discusses various types of alcohols, their properties, and classification, including solubility in water and boiling points affected by molecular size and hydrogen bonding. It also covers oxidation reactions of primary and secondary alcohols to aldehydes and ketones, as well as the production of ethanol through fermentation and chemical methods. Finally, it describes the formation and uses of esters derived from alcohols and carboxylic acids.









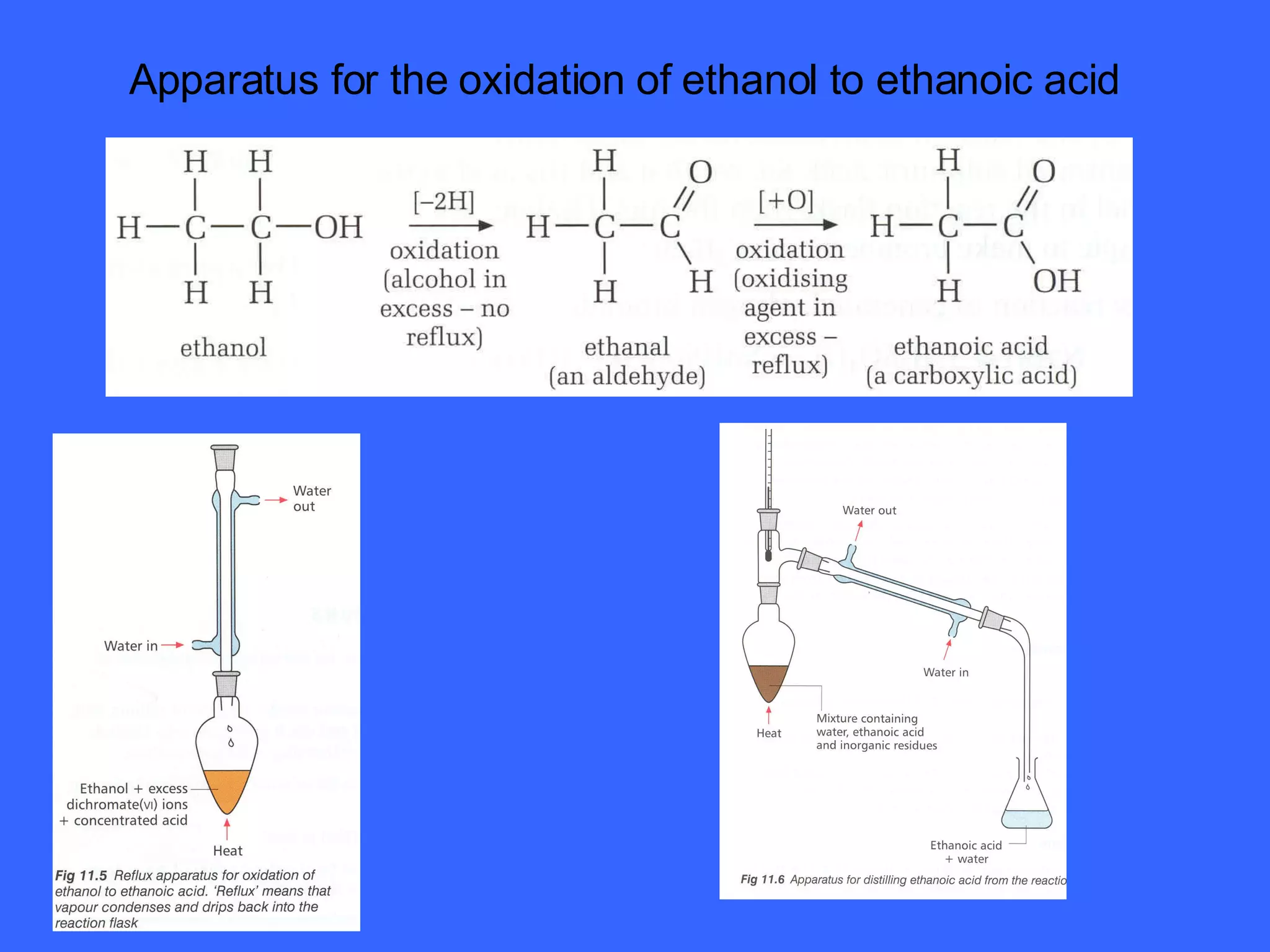
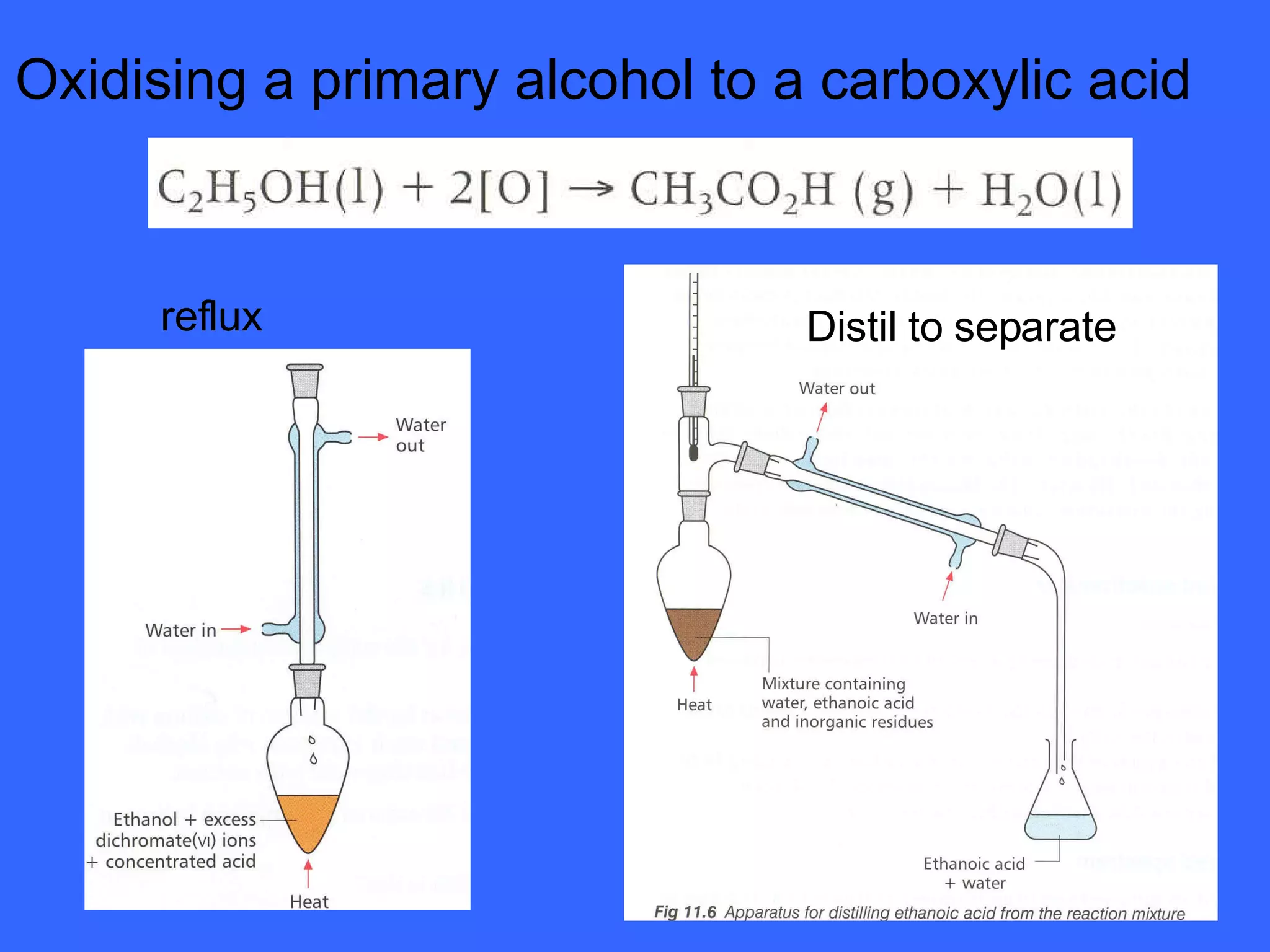
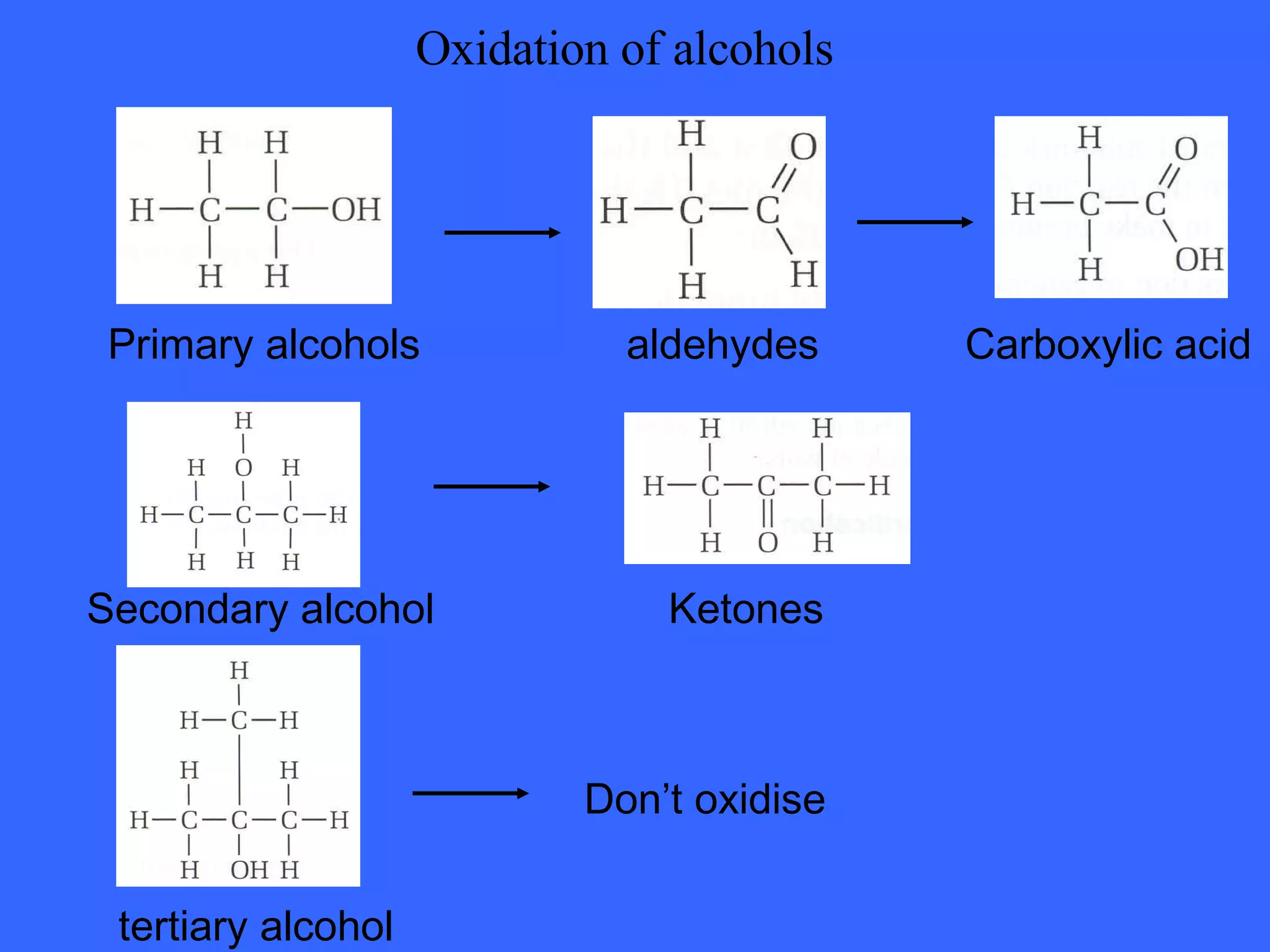
![OXIDATION OF PRIMARY ALCOHOLS Primary alcohols are easily oxidised to aldehydes e.g. CH 3 CH 2 OH(l) + [O] ——> CH 3 CHO(l) + H 2 O(l) ethanol ethanal it is essential to distil off the aldehyde before it gets oxidised to the acid CH 3 CHO(l) + [O] ——> CH 3 COOH(l) ethanal ethanoic acid Practical details the alcohol is dripped into a warm solution of acidified K 2 Cr 2 O 7 aldehydes have low boiling points - no hydrogen bonding - they distil off immediately if it didn’t distil off it would be oxidised to the equivalent carboxylic acid to oxidise an alcohol straight to the acid, reflux the mixture compound formula intermolecular bonding boiling point ETHANOL C 2 H 5 OH HYDROGEN BONDING 78°C ETHANAL CH 3 CHO DIPOLE-DIPOLE 23°C ETHANOIC ACID CH 3 COOH HYDROGEN BONDING 118°C](https://image.slidesharecdn.com/alcohol-powerpoint-2406/75/Alcohol-powerpoint-11-2048.jpg)