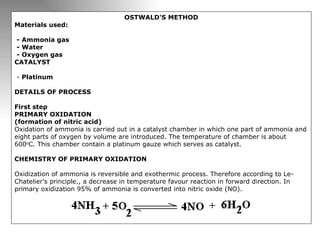
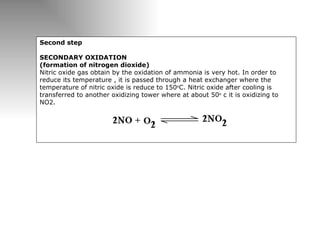
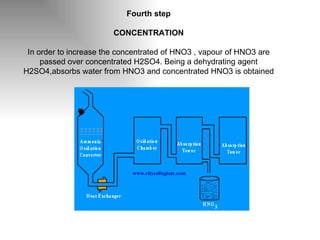
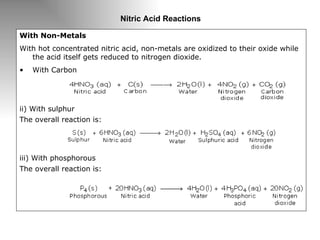
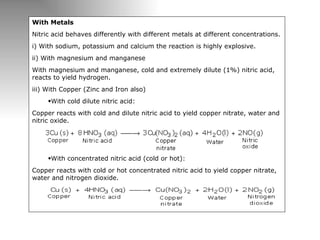
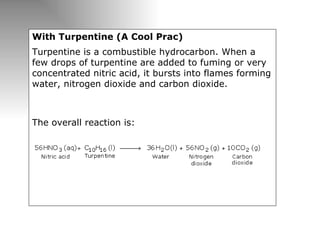
Nitric acid is a strong acid that is colorless as a pure liquid but commercial samples may appear yellowish. It is highly corrosive and a strong oxidizer. Nitric acid is produced industrially via the Ostwald process, which involves ammonia oxidation over a platinum catalyst in three steps: primary oxidation to nitric oxide, secondary oxidation to nitrogen dioxide, and absorption of nitrogen dioxide in water to form nitric acid. Nitric acid has many industrial and laboratory uses including fertilizer and explosive production.