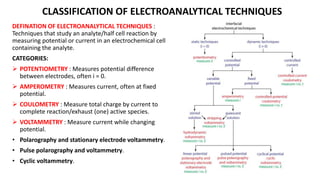
A Daniell cell is a galvanic cell that uses the spontaneous redox reaction between zinc and copper(II) ions to produce an electric current. It consists of an outer copper vessel containing a copper electrode in a copper(II) sulfate solution and an inner amalgamated zinc electrode in a zinc sulfate solution, separated by a salt bridge. Electrons flow from the zinc anode through the external circuit to the copper cathode. The cell reactions involve the oxidation of zinc to zinc ions at the anode and the reduction of copper ions to copper at the cathode.



![The associated chemical equations and the overall cell reaction are provided
below
• Reaction at Cathode: [Na+ + e– → Na] x 2
• Reaction at Anode: 2Cl– → Cl2 + 2e–
• Cell Reaction: 2NaCl → 2Na + Cl2
• Thus, molten sodium chloride can be subjected to electrolysis in an electrolytic cell to
generate metallic sodium and chlorine gas as the products.
• Applications of Electrolytic Cells
• The primary application of electrolytic cells is for the production of oxygen gas and
hydrogen gas from water.
• They are also used for the extraction of aluminium from bauxite.
• Another notable application of electrolytic cells is in electroplating, which is the process of
forming a thin protective layer of a specific metal on the surface of another metal.
• The electrorefining of many non-ferrous metals is done with the help of electrolytic cells.](https://image.slidesharecdn.com/galvanicorvoltaiccell-230426174320-da8e1ee1/85/GALVANIC-AND-ELECTROLYTIC-CELL-5-320.jpg)