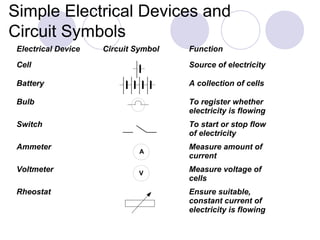
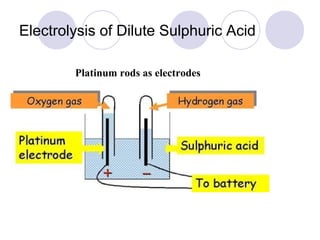
1. Electrolysis is the process of using electricity to cause non-spontaneous chemical changes.
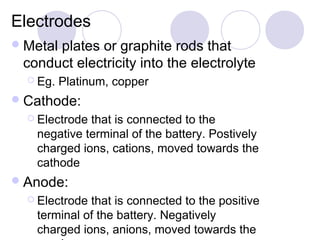
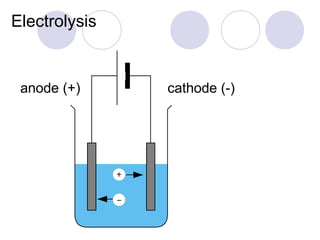
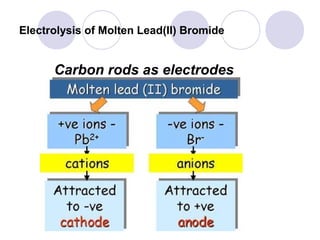
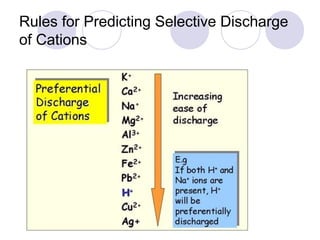
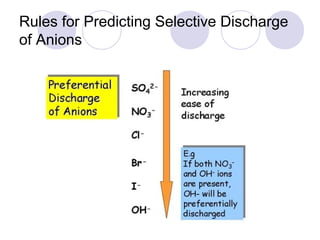
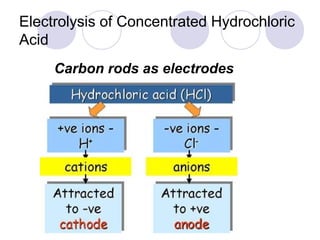
2. During electrolysis, ions migrate towards the oppositely charged electrode - cations move towards the cathode and anions move towards the anode.
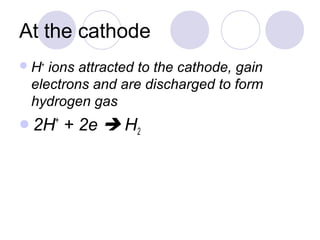
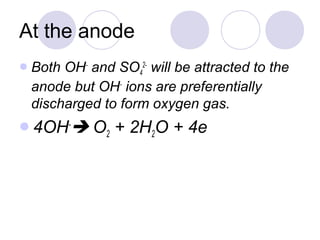
3. At the cathode, cations gain electrons and are reduced. At the anode, anions lose electrons and are oxidized.
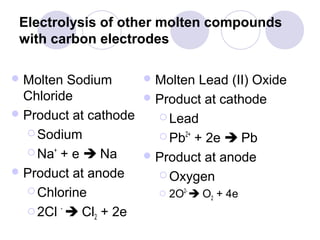
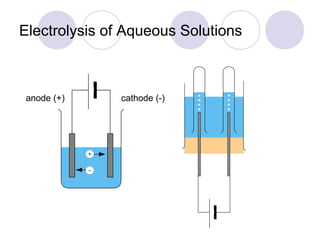
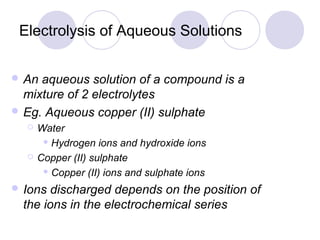
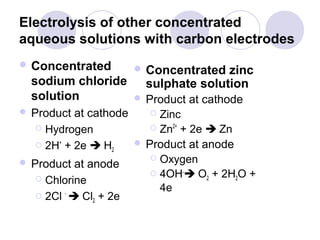
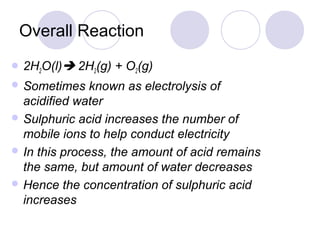
4. The products of electrolysis depend on the electrolyte. Molten salts yield elements, while aqueous solutions yield hydrogen or oxygen along with other possible products.