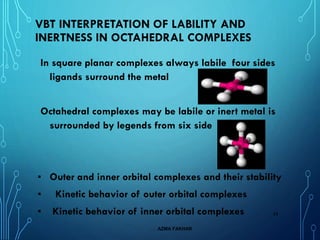
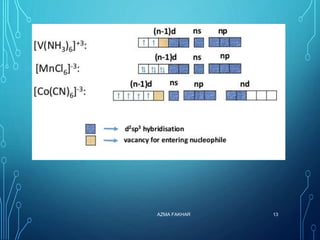
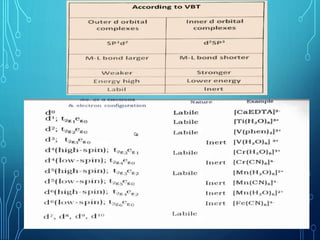
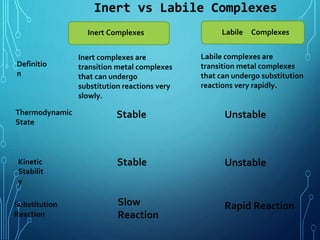
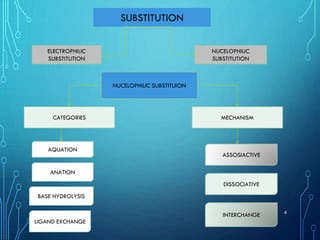
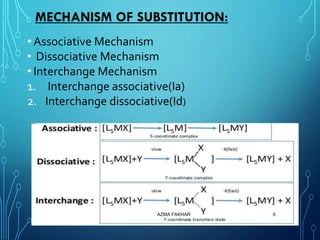
This document provides an overview of substitution reactions and stability of complexes. It discusses labile complexes, which undergo substitution reactions rapidly, and inert complexes, which react slowly. Stability is divided into kinetic stability, referring to lability and inertness, and thermodynamic stability, whether a complex is stable or unstable. Factors like metal ion charge, size, and electron configuration determine if a complex is kinetically labile or inert.





![STABILITY OF COMPLEXES:
Stability is of two types Kinetic stability and thermodynamics
In both type of stability is discussed whether a complex is stable or not.
Kinetic stability has inert complexes and labile complexes AND
in Thermodynamics stability whether the complex is stable or unstable .
Consider the reaction: M + nL ⇌ MLn ;
βn =[MLn]/[M][L]n where βn is formation constant of the complex. The
higher values of βn indicate it’s higher thermodynamic stability of the
complex. Thus it gives measure of the extent to which the reaction
proceeds but it cannot say anything about the speed with which
equilibrium is attained.
AZMA FAKHAR 8](https://image.slidesharecdn.com/presenttionppts-210331105030/85/labile-and-inert-complexe-stable-and-unstable-complex-8-320.jpg)