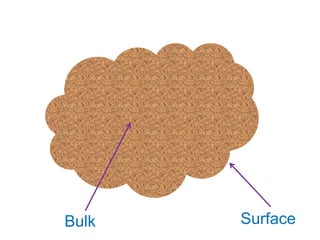
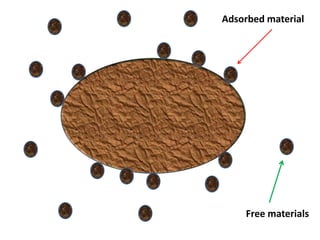
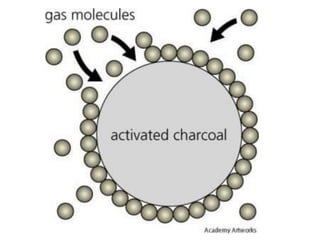
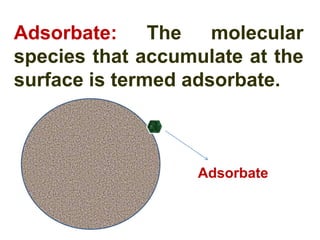
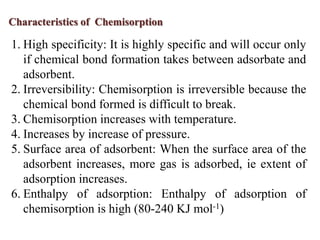
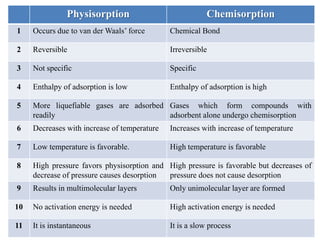
The document discusses adsorption, which is the accumulation of molecules on the surface of solids or liquids. It defines key terms like adsorbate, adsorbent, desorption, and occlusion. The document also distinguishes between physisorption and chemisorption, and notes factors that influence adsorption like surface area, temperature, and pressure. Some applications of adsorption are mentioned as well, such as in gas masks, vacuum production, water softening, catalysis, petroleum refining, and chromatography.