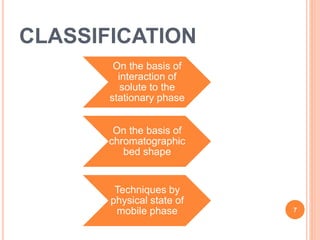
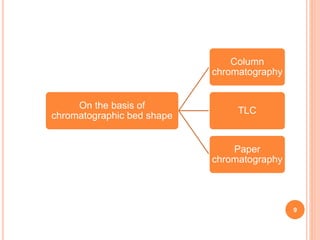
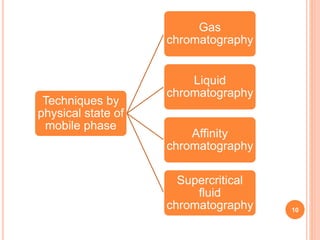
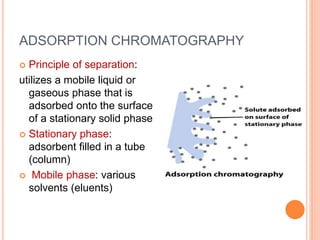
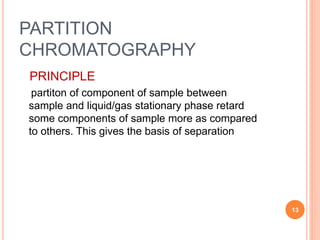
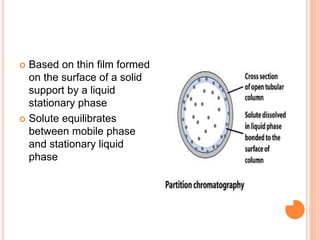
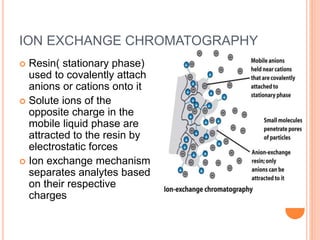
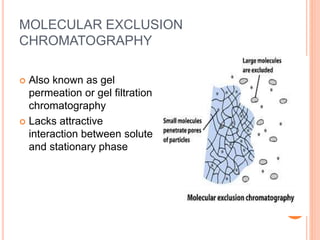
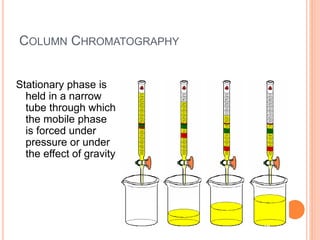
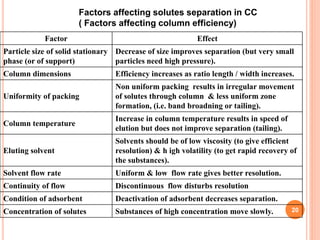
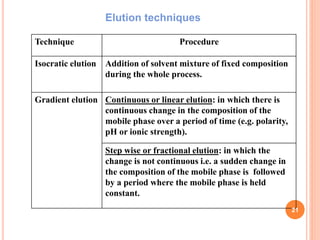
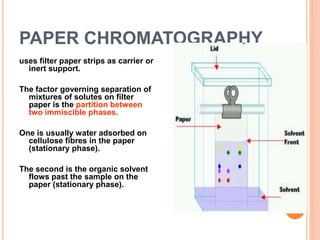
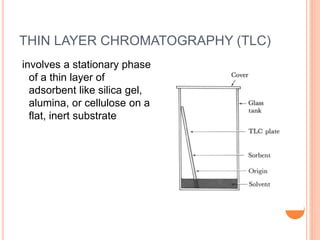
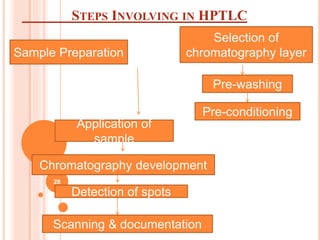
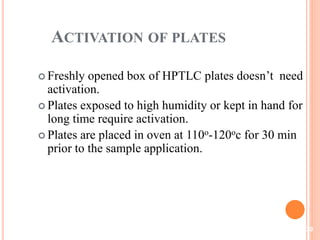
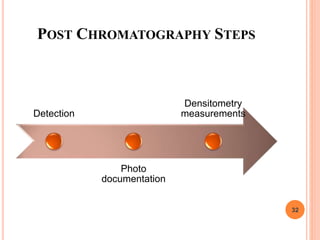
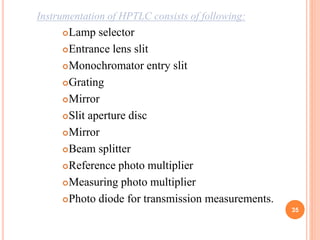
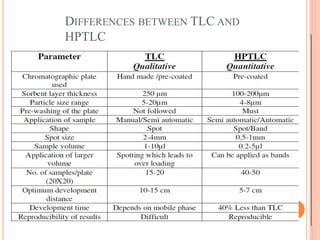
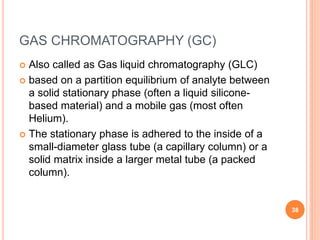
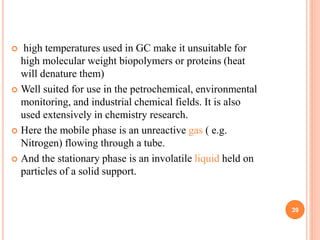
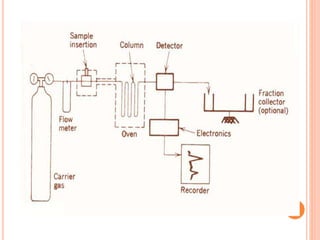
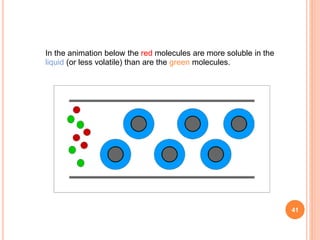
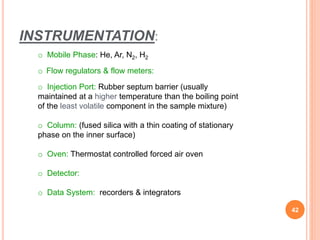
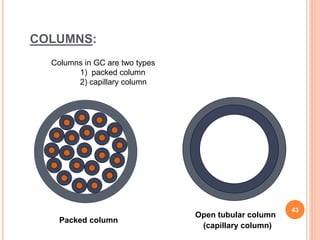
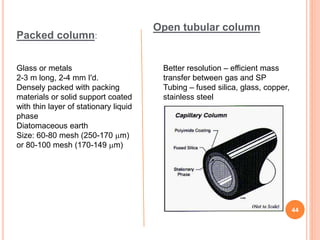
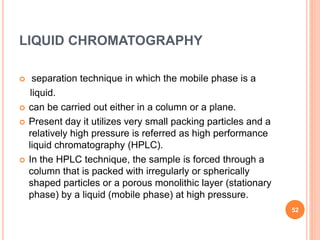
Chromatography is a technique used to separate and identify the components of a mixture. It works by allowing molecules to distribute themselves between a stationary and mobile phase, so that molecules that interact more with the mobile phase move faster. Chromatographic techniques can be classified based on the interaction with the stationary phase or physical state of the mobile phase. Key techniques include adsorption, partition, ion exchange, exclusion, gas, liquid, and thin layer chromatography. Proper sample preparation and development conditions are important for achieving optimal separation and resolution of components in the mixture.






















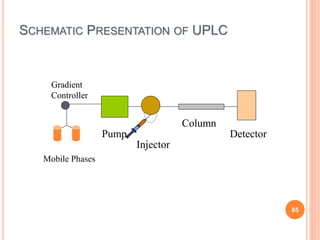
![UPLC
In 2004, further advances in instrumentation and
column technology were made to achieve very
significant increase in:
RESOLUTION
SPEED
SENSITIVITY
Increase separation EFFICIENCY
Columns with smaller particles [<1.7um]
Mobile phase delivery is done at >15,000psi
62](https://image.slidesharecdn.com/seminar-151119020300-lva1-app6892/85/chromatographic-techniques-62-320.jpg)