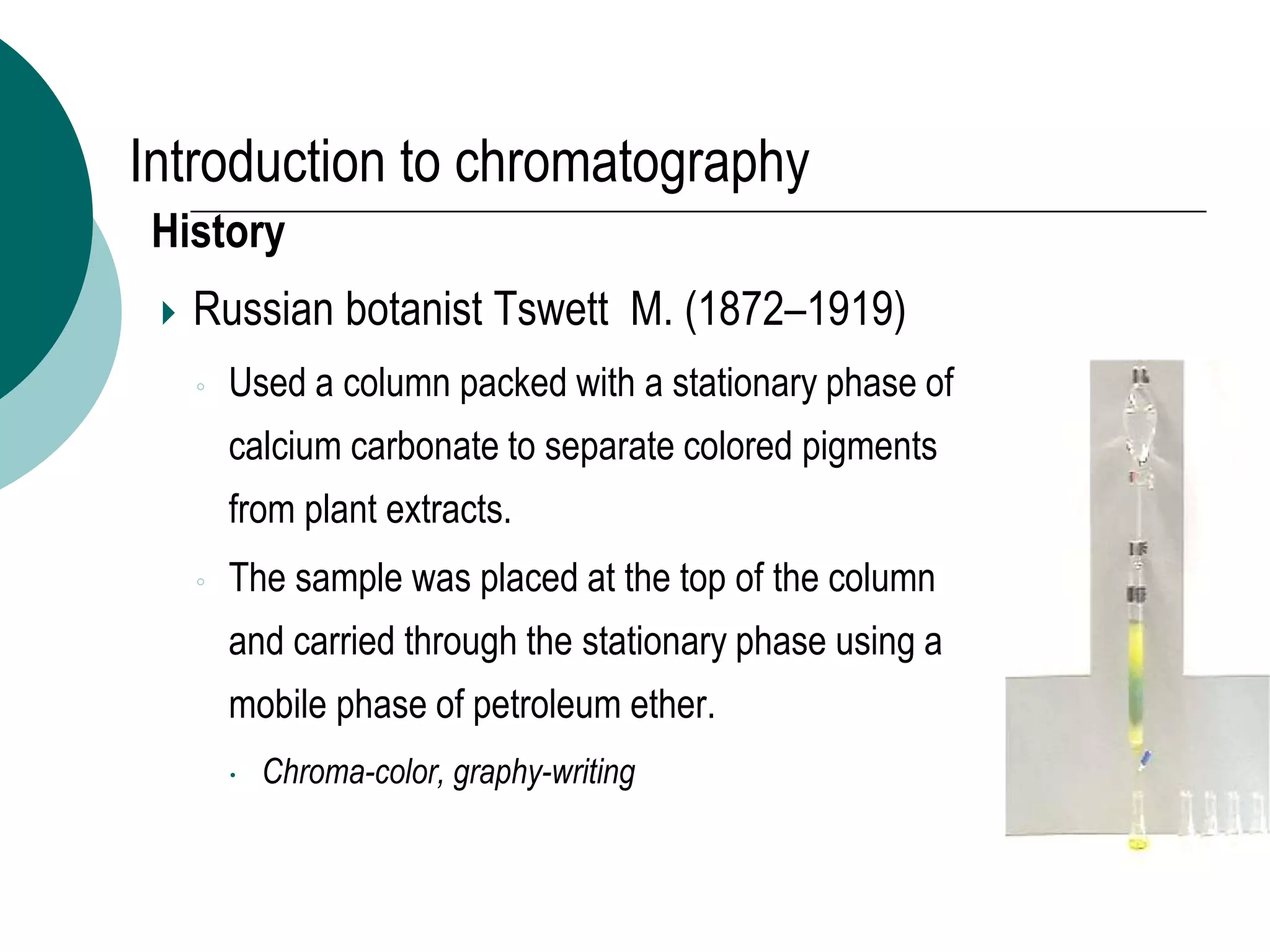
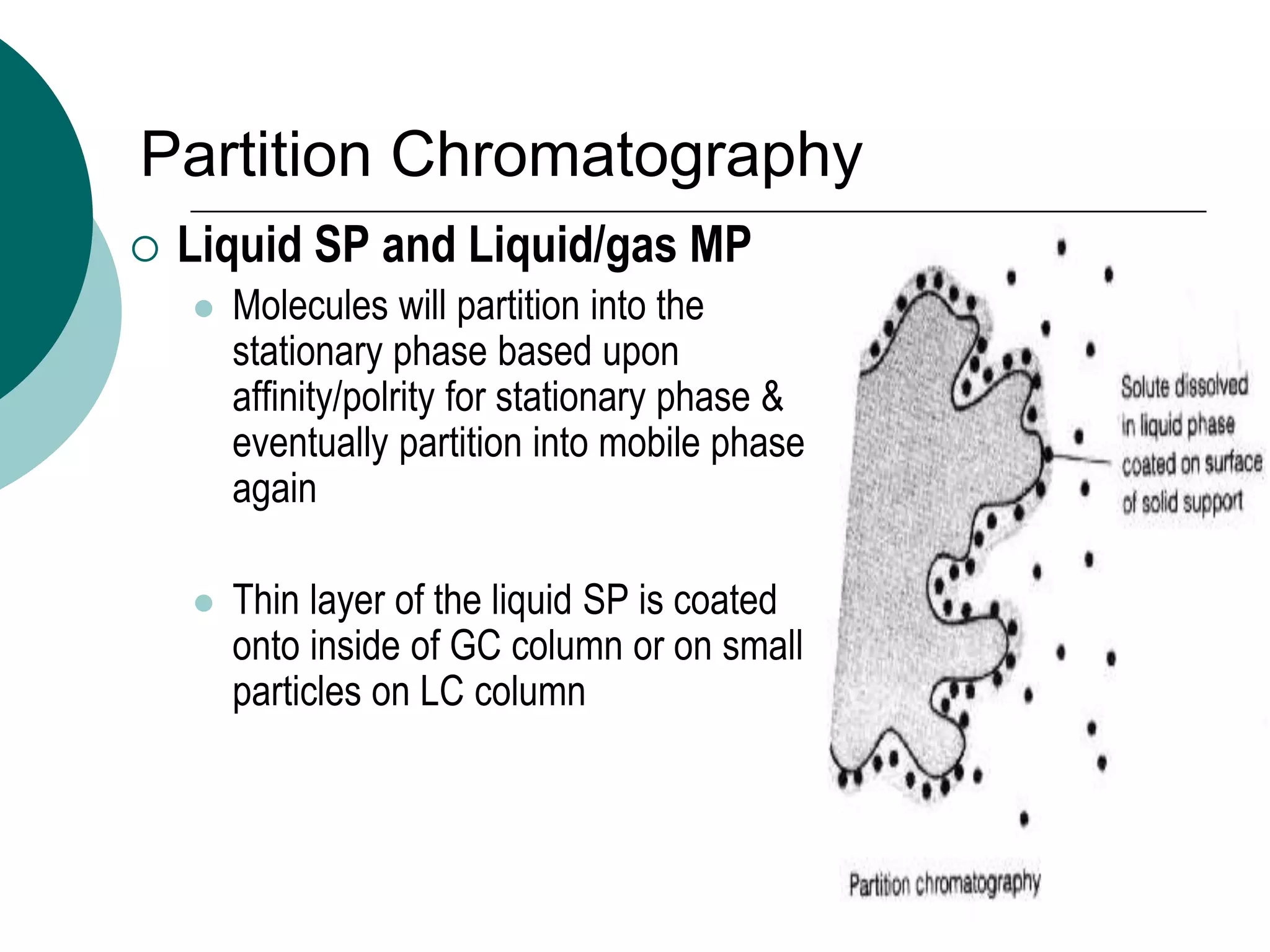
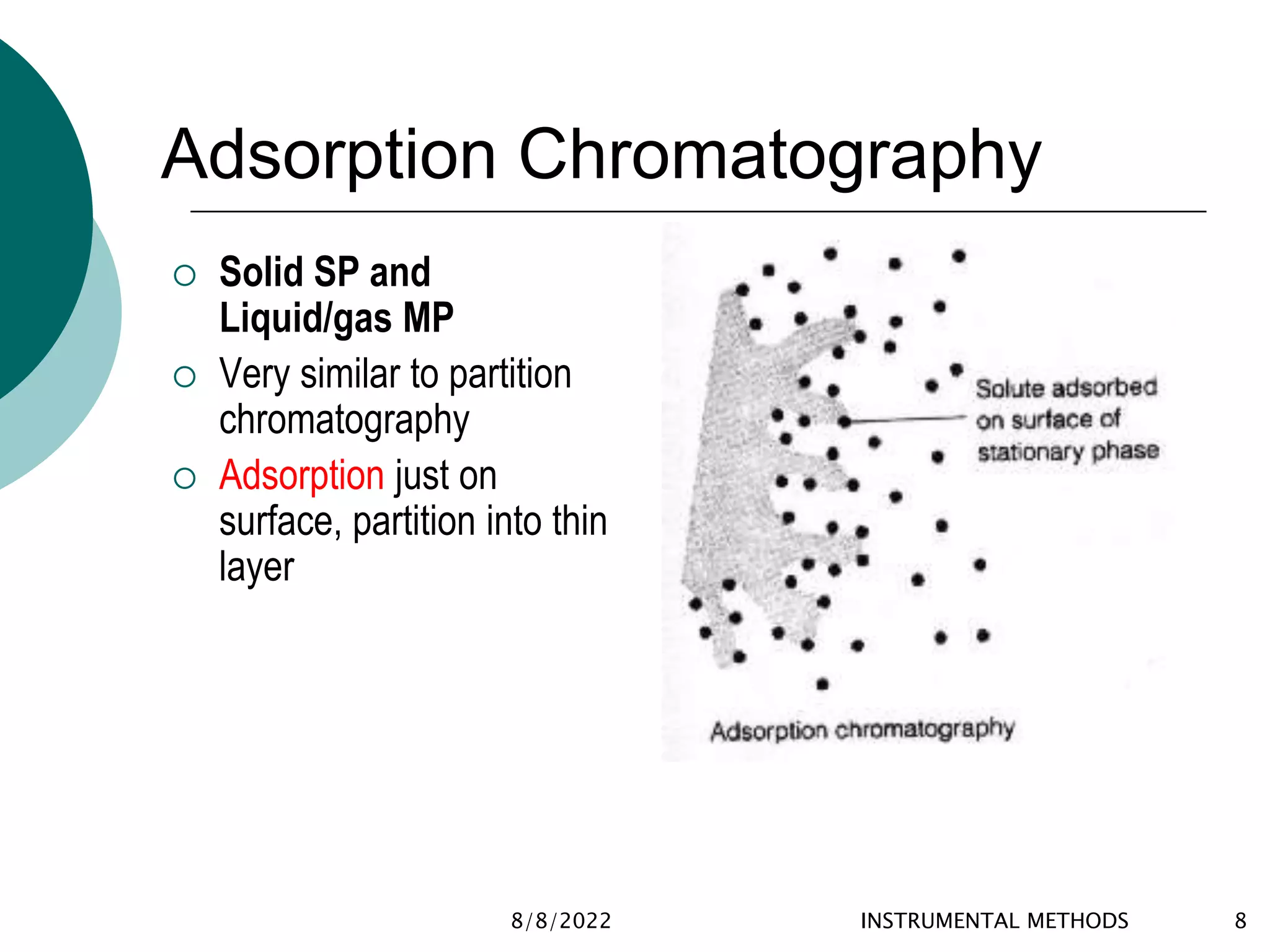
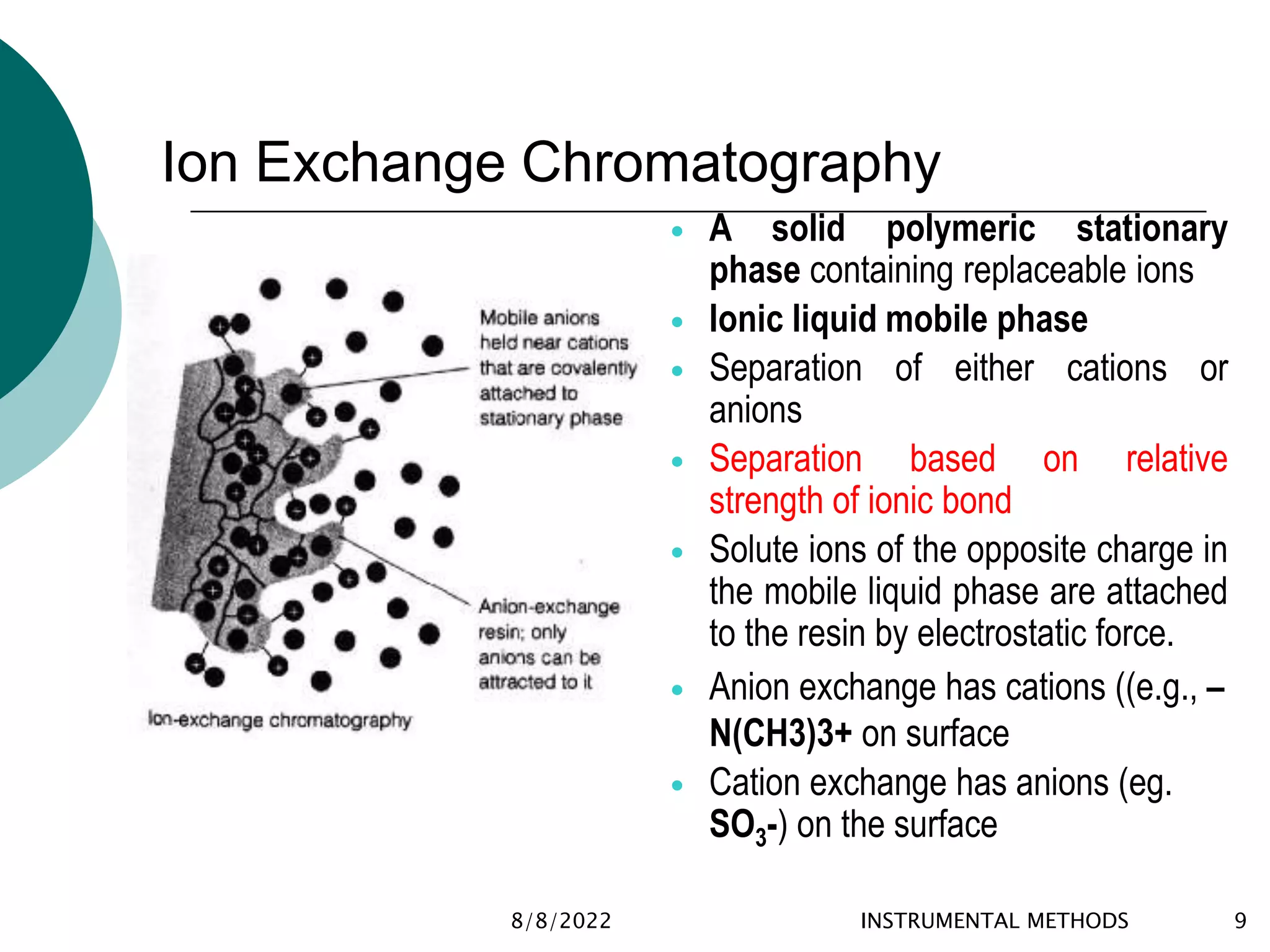
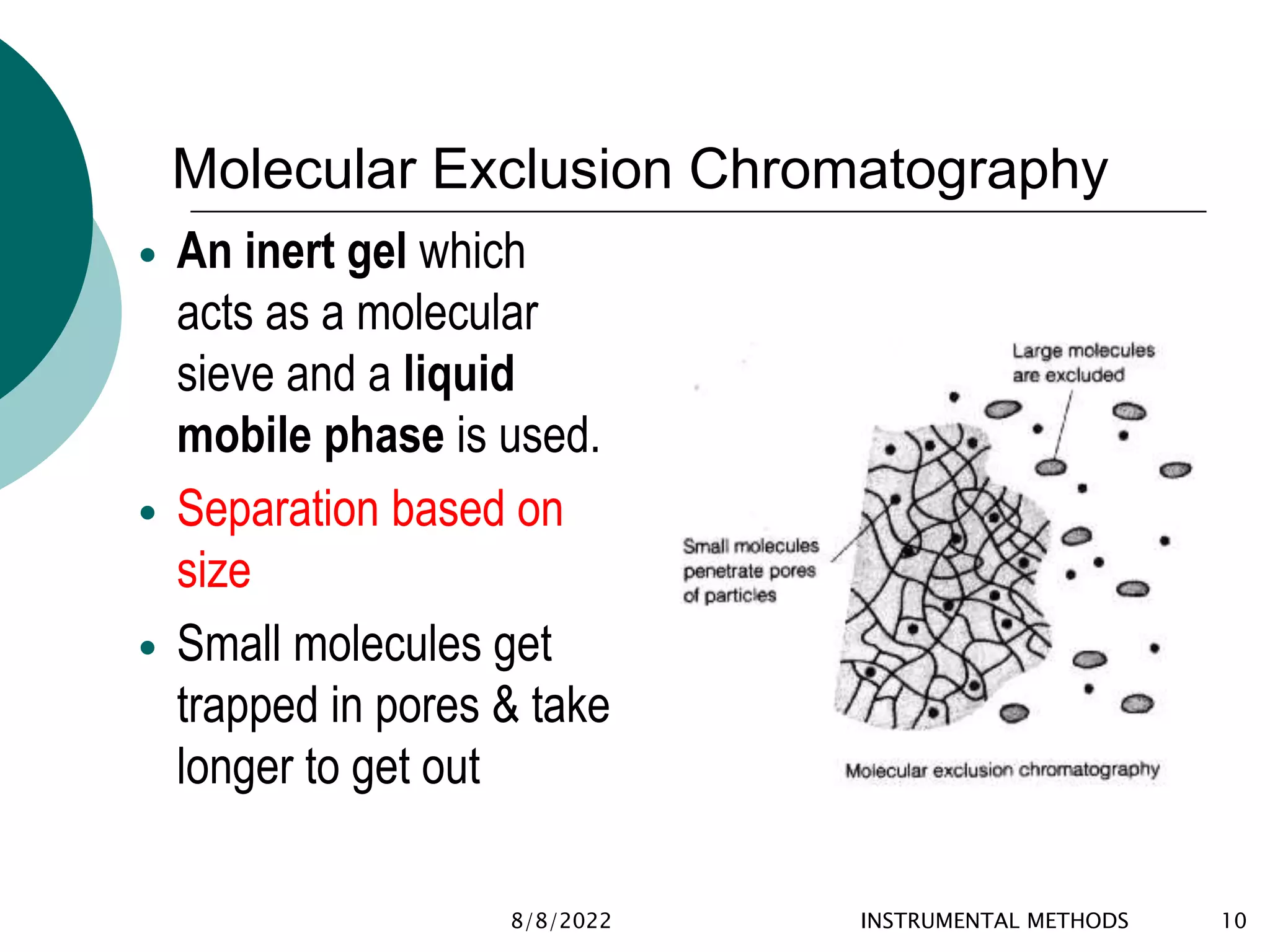
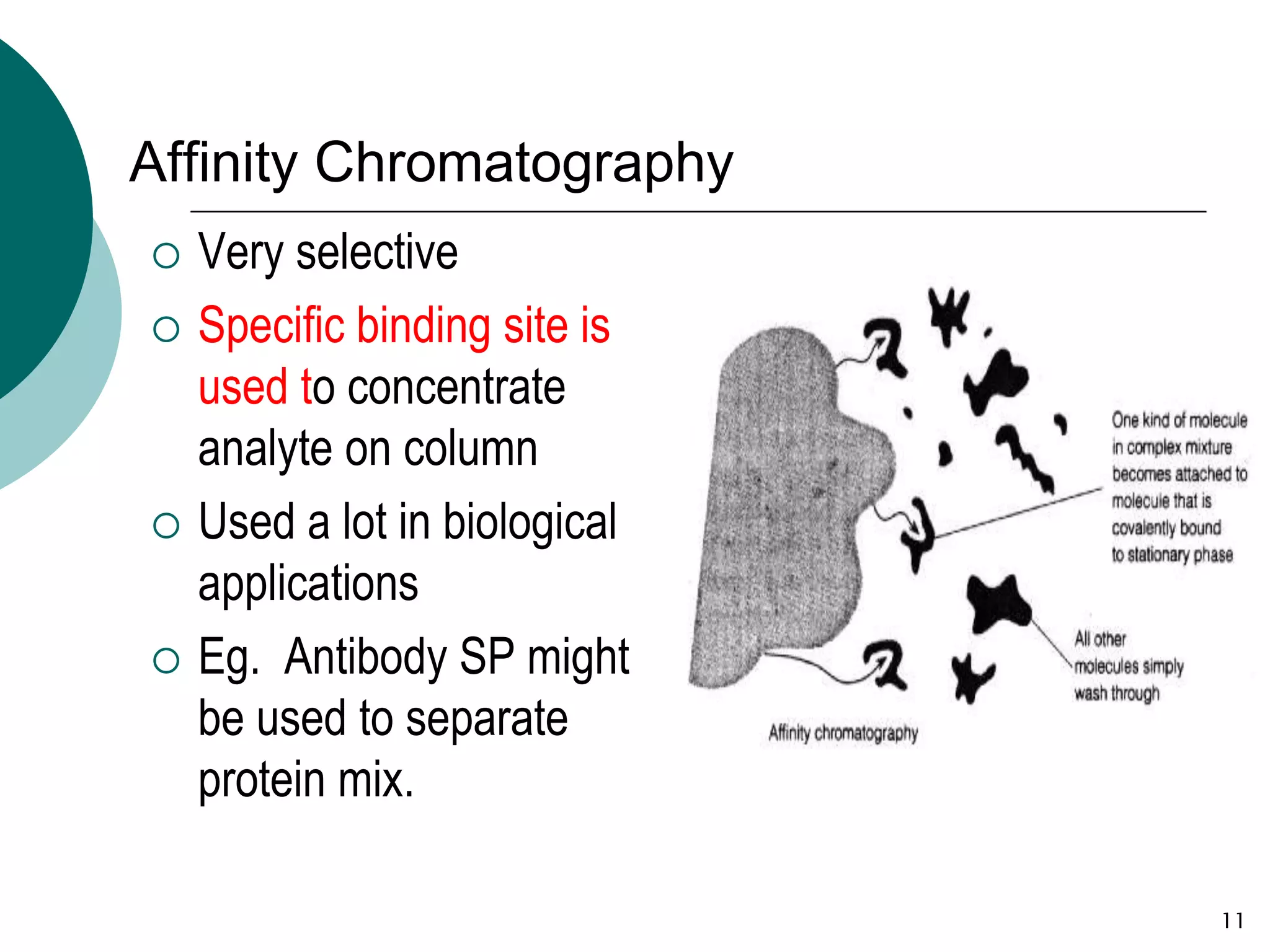
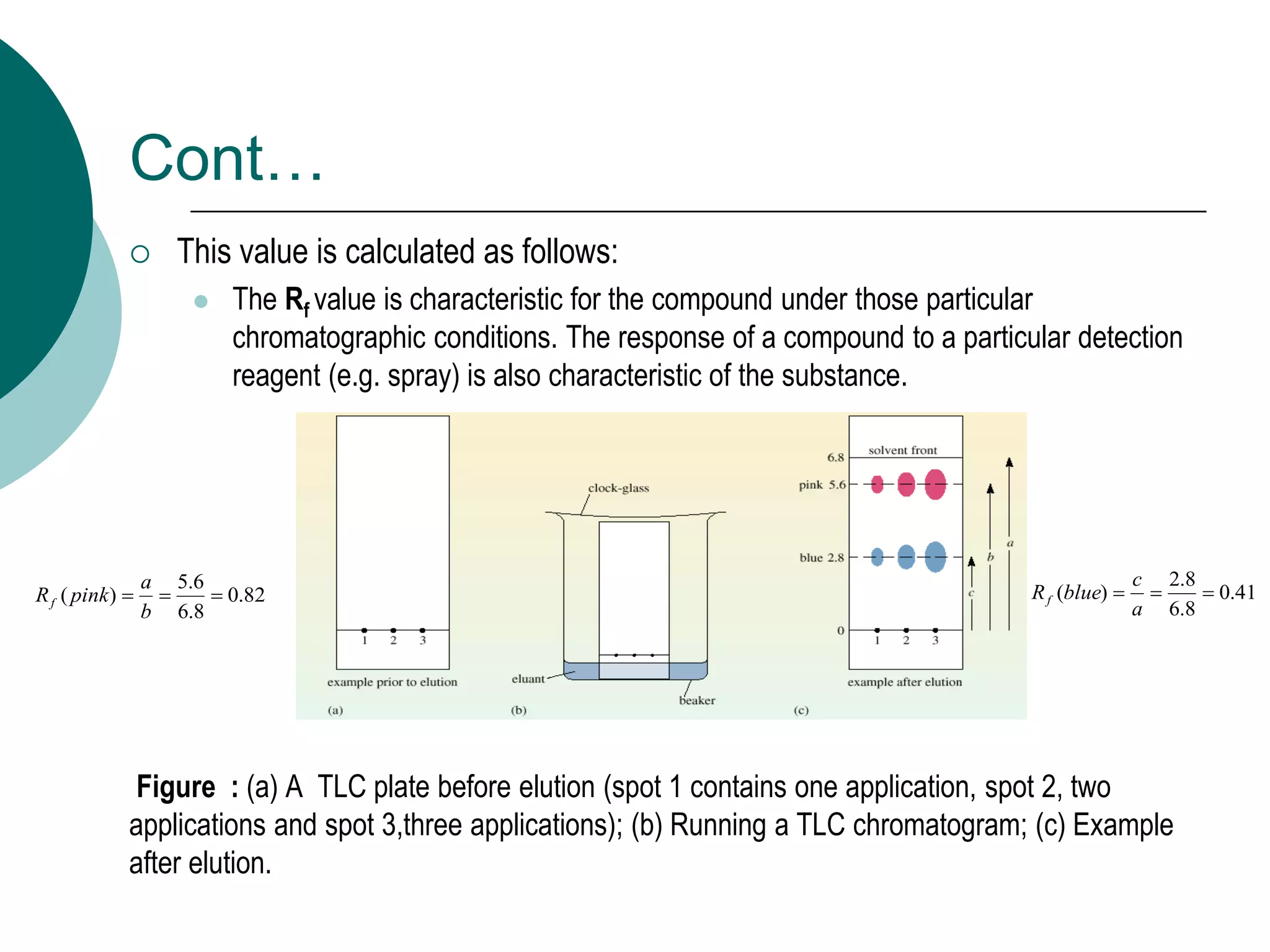
This document provides an introduction to chromatography, including its history and essential features. It discusses the basic components and process of chromatography, including the stationary and mobile phases. It also describes different types of chromatography techniques based on the stationary phase, such as partition chromatography, adsorption chromatography, ion exchange chromatography, molecular exclusion chromatography, and affinity chromatography. Finally, it discusses applications of chromatography in qualitative analysis, quantitative analysis, and preparative purposes.