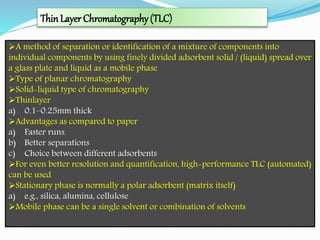
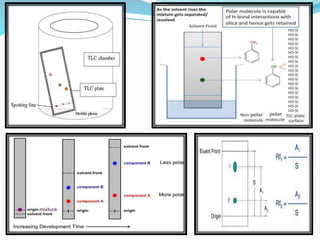
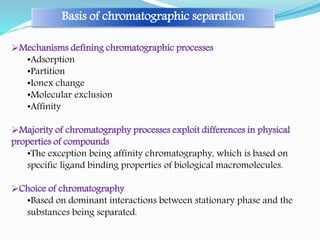
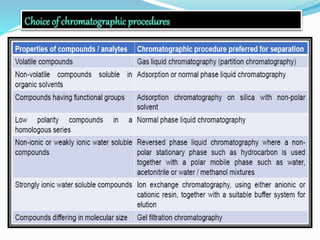
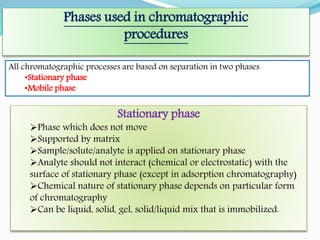
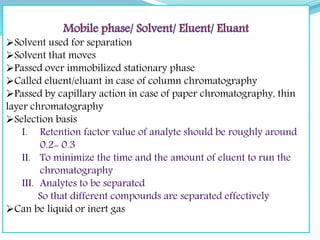
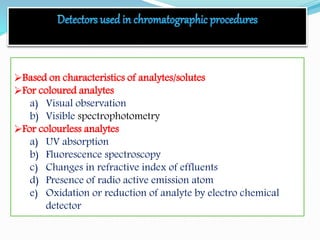
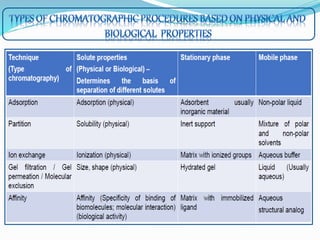
Chromatography is a technique used to separate chemical mixtures by exploiting differences in how components partition between a stationary and mobile phase. There are various types including adsorption, partition, ion exchange, and affinity chromatography. The document provides details on the basic principles, mechanisms, phases used, instrumentation, and common techniques like column chromatography, paper chromatography, thin layer chromatography, and gas chromatography.





![Steps
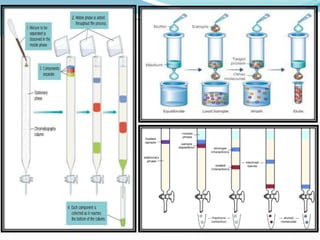
Packing of column with stationary phase
Loading of analyte mix
Passage of mobile phase
[Washing of unbound analytes in the mix and
elution of bound analytes in the mix by mobile phase]
Fraction collection
Detection of separated analytes
Columnchromatography](https://image.slidesharecdn.com/eadpptbysandeepkumar-210316111022/85/Chromatography-12-320.jpg)

![Stationary phase attached to suitable matrix is packed in column and equilibrated
[Uniform packing with no cracks or channels or bubbles]
[Stationary phase should be wet with the solvent]
[Supported at bottom by glasswool]
[Stop cock closed]
↓
Small volume (thin lamella) of analyte mix placed on stationary phase and allowed to
enter the column
↓
Mobile phase constantly passed through column
[By gravity feed / By pumping system / By applied gas pressure]
[Stop cock opened]
[Stationary phase should not be disturbed upon addition of mobile phase]
[Mobile phase added repeatedly as many times as needed throughout the process]
↓
Different solutes in the mix move through column, get separated and chromatogram
developed by flowing mobile phase
[Different washing and elution steps may be required]
↓](https://image.slidesharecdn.com/eadpptbysandeepkumar-210316111022/85/Chromatography-14-320.jpg)
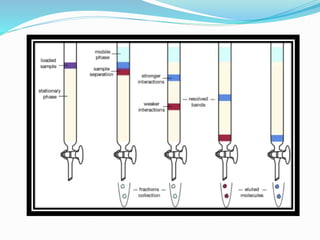
![Separated solutes appear in the liquid leaving the column (eluate / effluent) when
particular volumes of mobile phase have passed through column
↓
Eluate collected as discrete fractions using automatic collector
[Component analytes thus purified from analyte mix]
↓
Separated components are identified by testing aliquots of each fraction by
spectrum measurements, chemical tests, radioactivity, etc., depending upon the
properties of analytes](https://image.slidesharecdn.com/eadpptbysandeepkumar-210316111022/85/Chromatography-15-320.jpg)
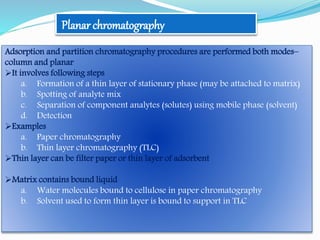




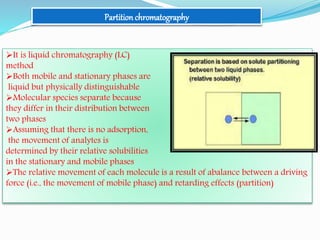

![Stationary phase–polar– e.g., alkylnitrile, alkylamine bonded to silica
Mobile phase–relatively non polar–e.g., organic solvent as hydrocarbons in
combination with ethers, esters and chlorinated solvents; hexane, heptane,
dichloromethane, ethylacetate
When the solvent or gradient of solvents is passed, the less polar components
will be eluted faster than the more
polar ones
Order of elution–non polar > polar [the least
polar is eluted first and the most polar last]](https://image.slidesharecdn.com/eadpptbysandeepkumar-210316111022/85/Chromatography-27-320.jpg)