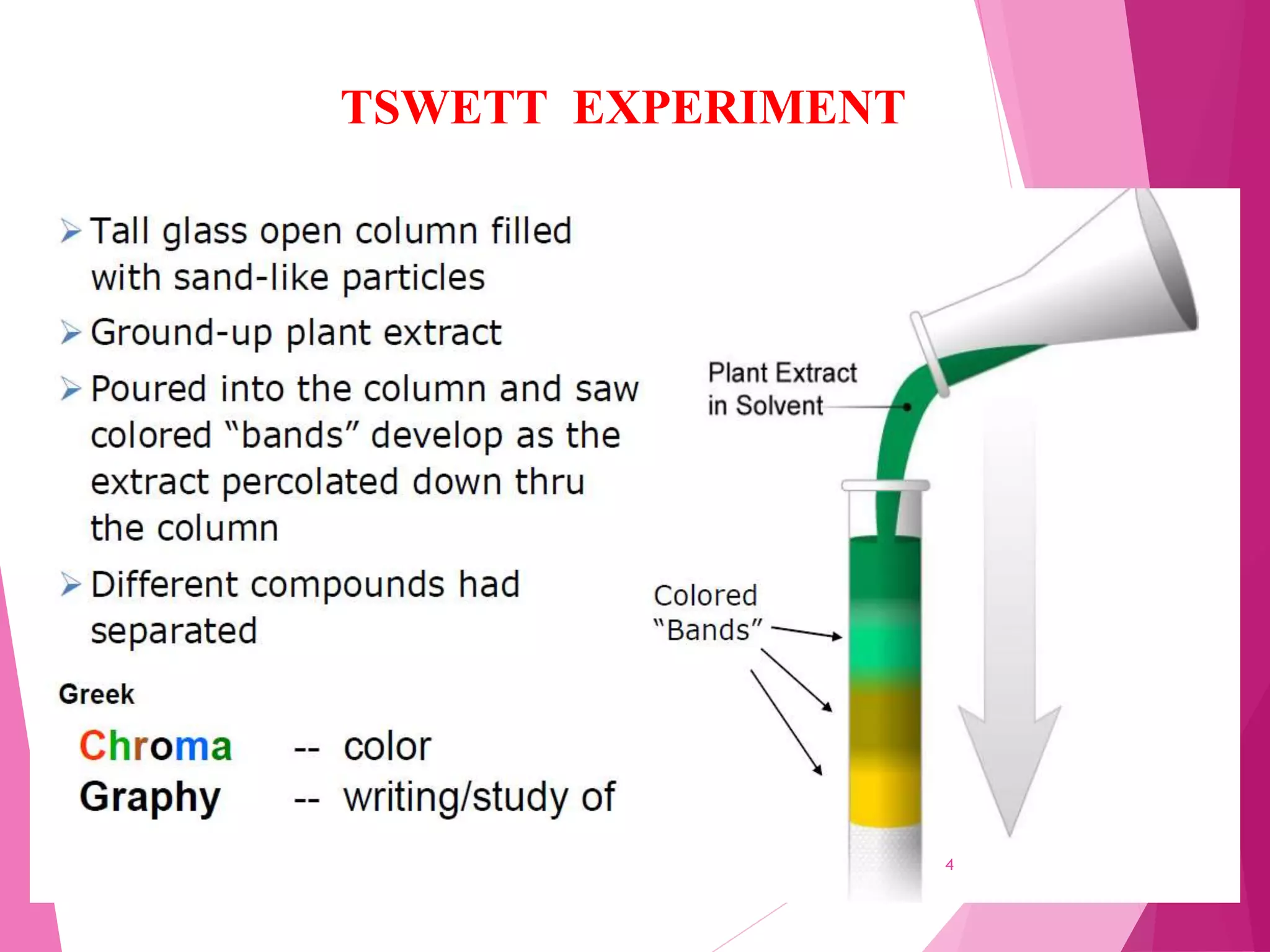
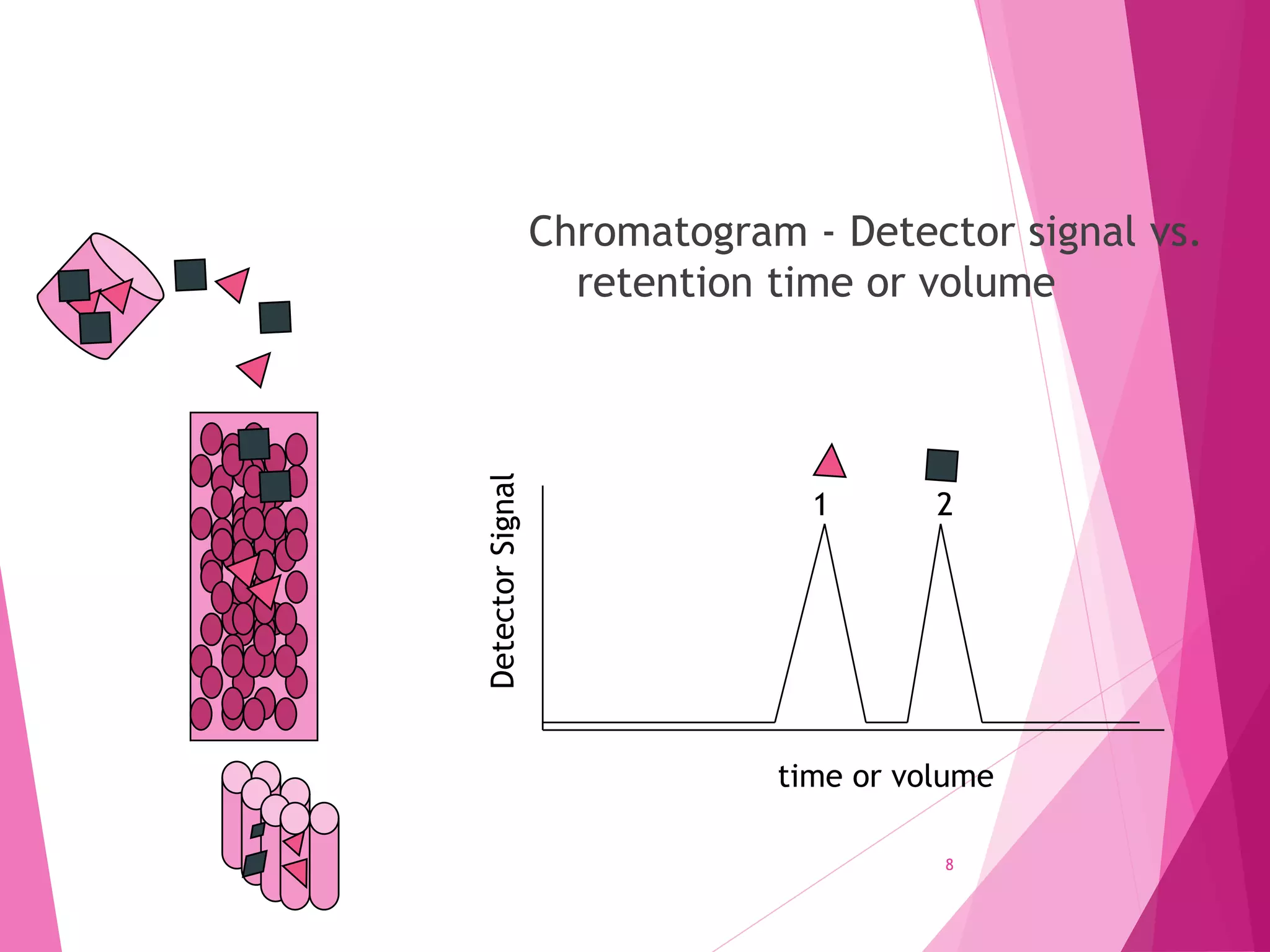
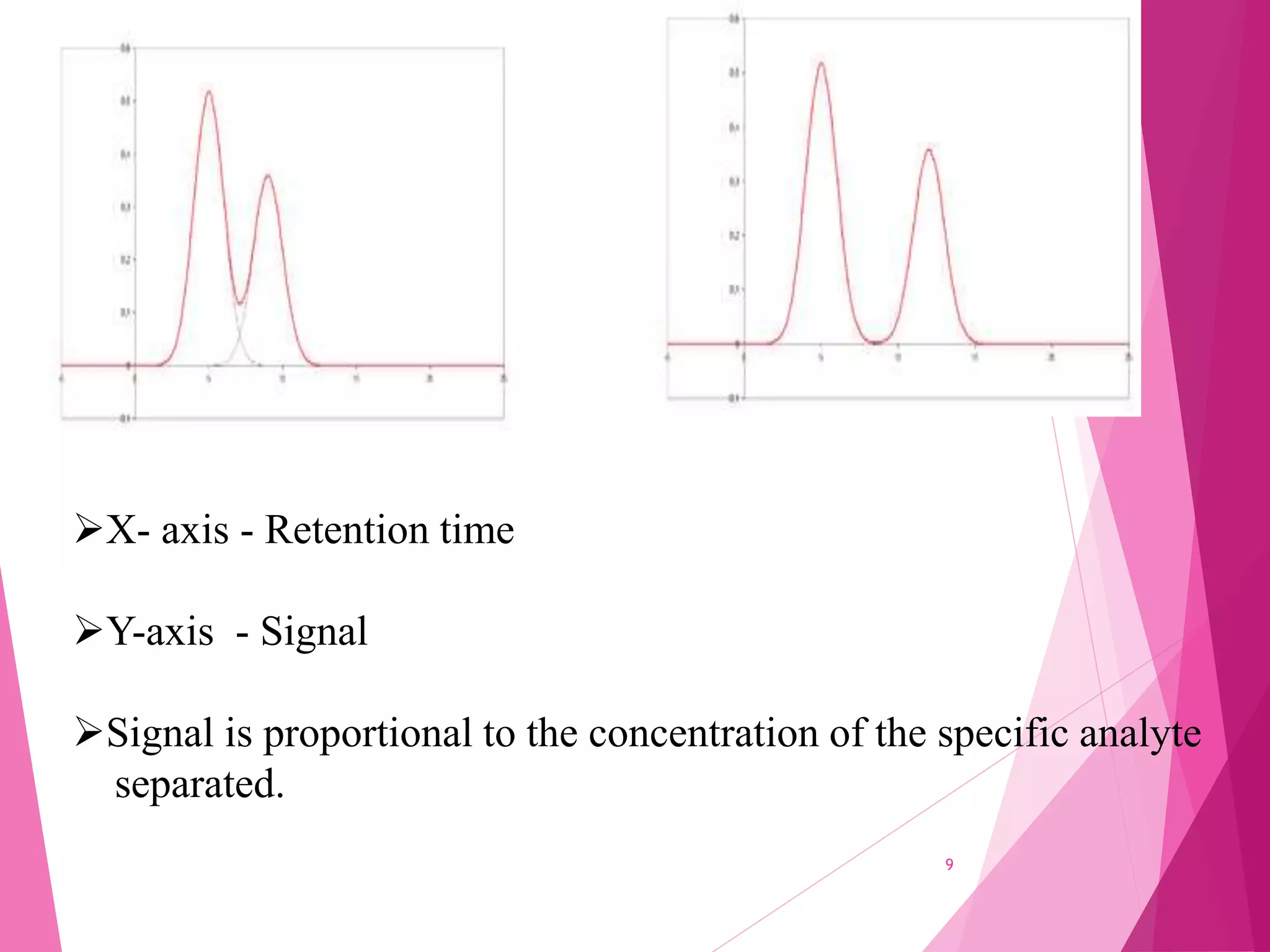
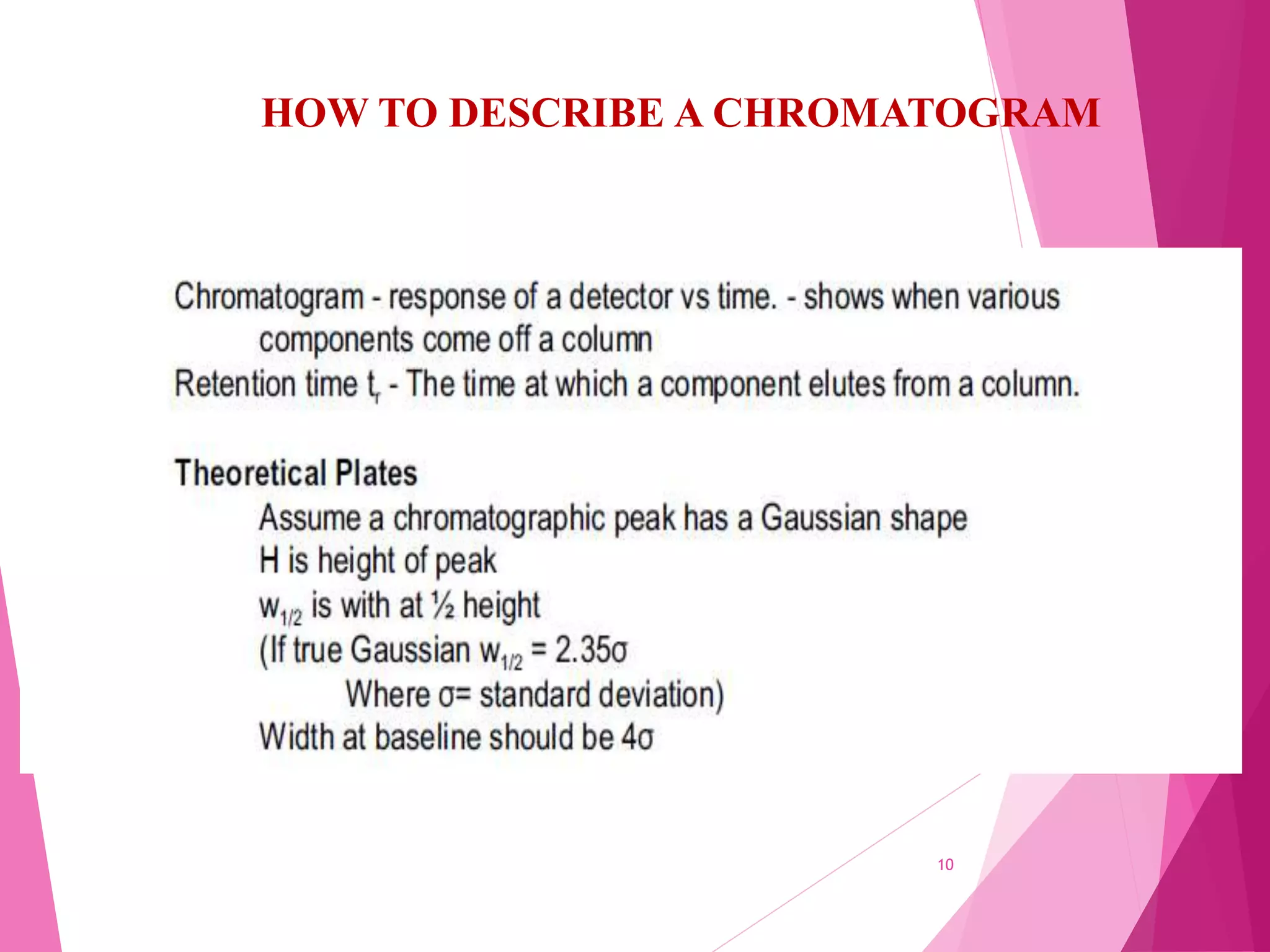
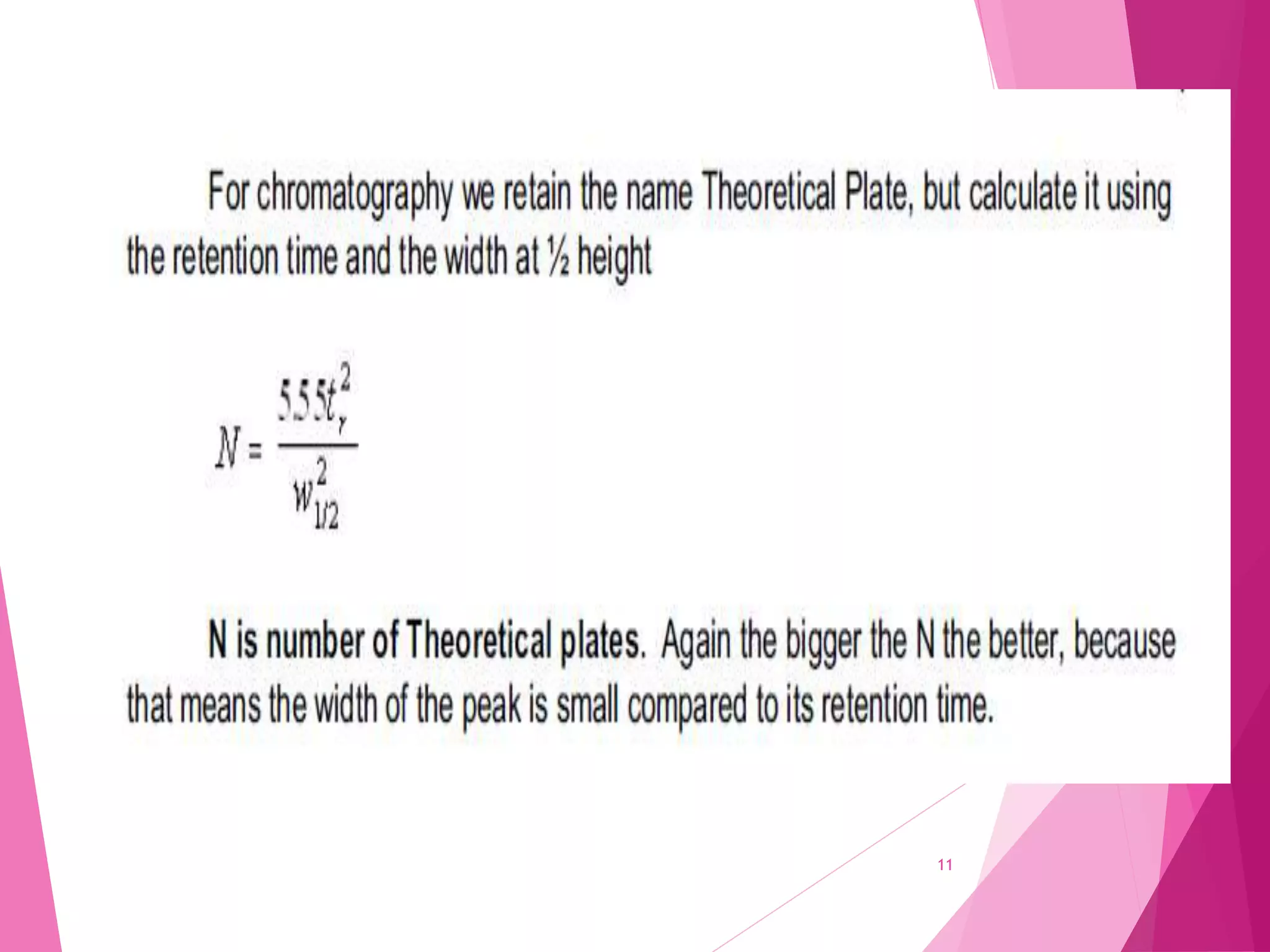
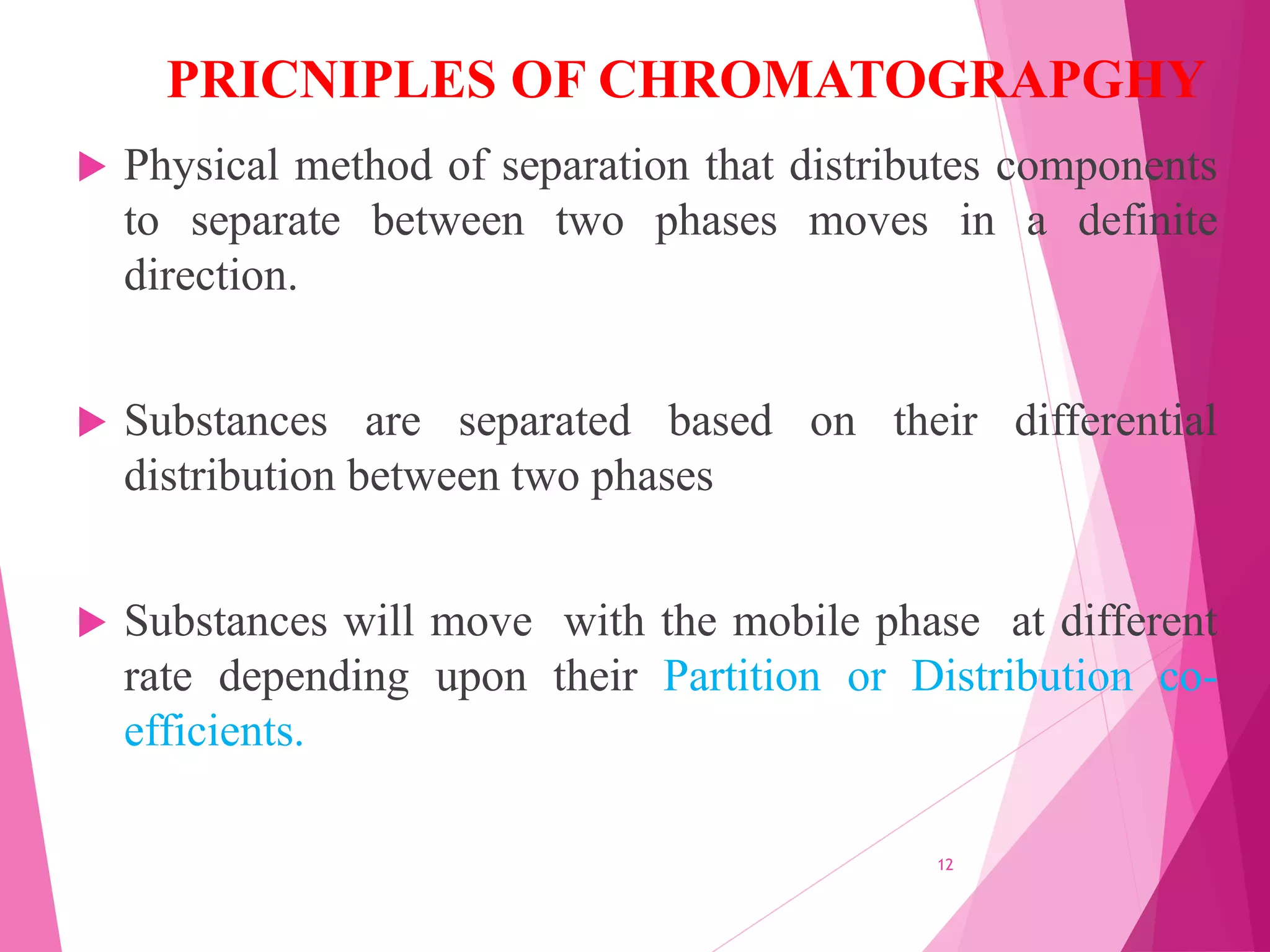
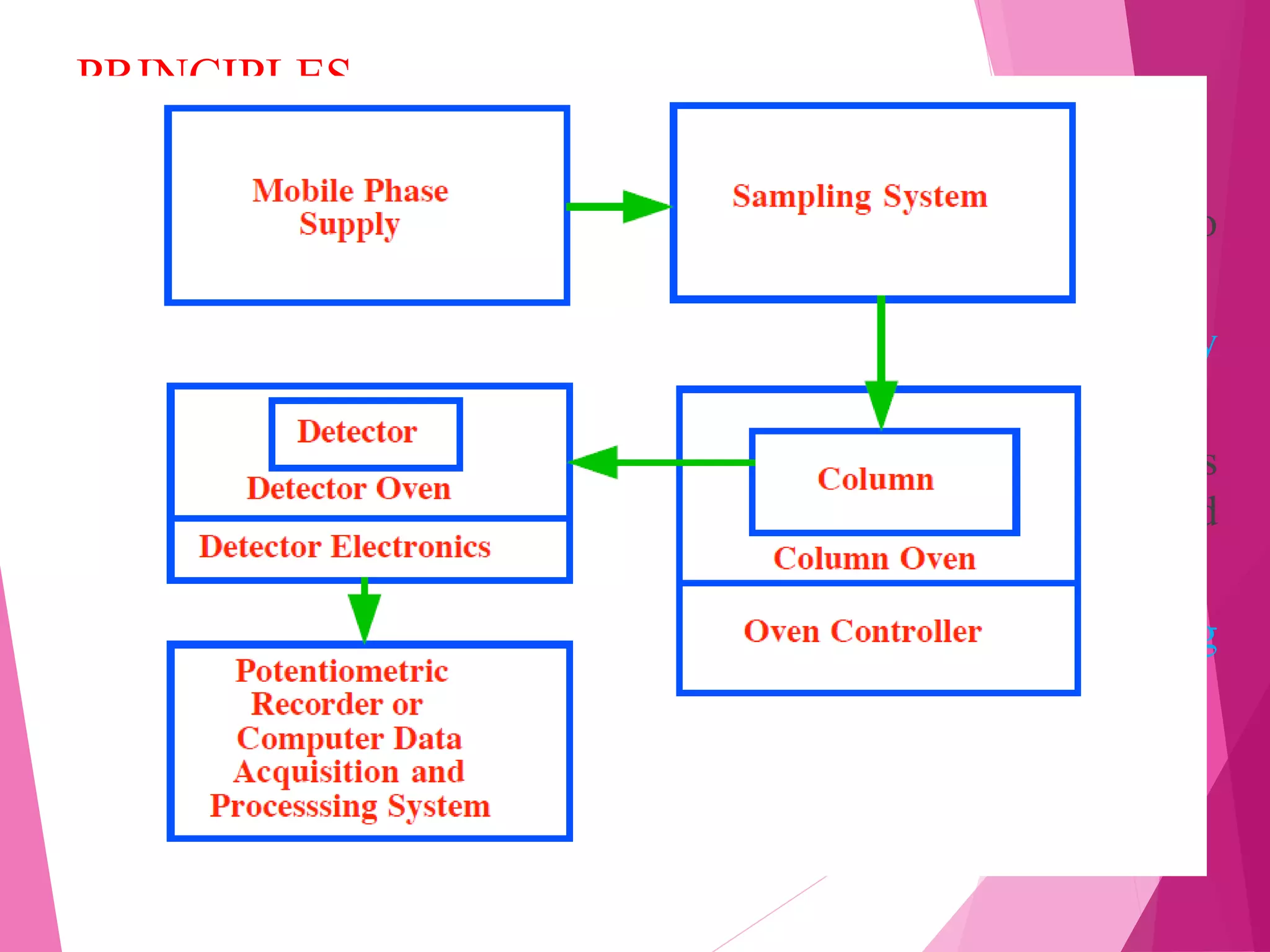
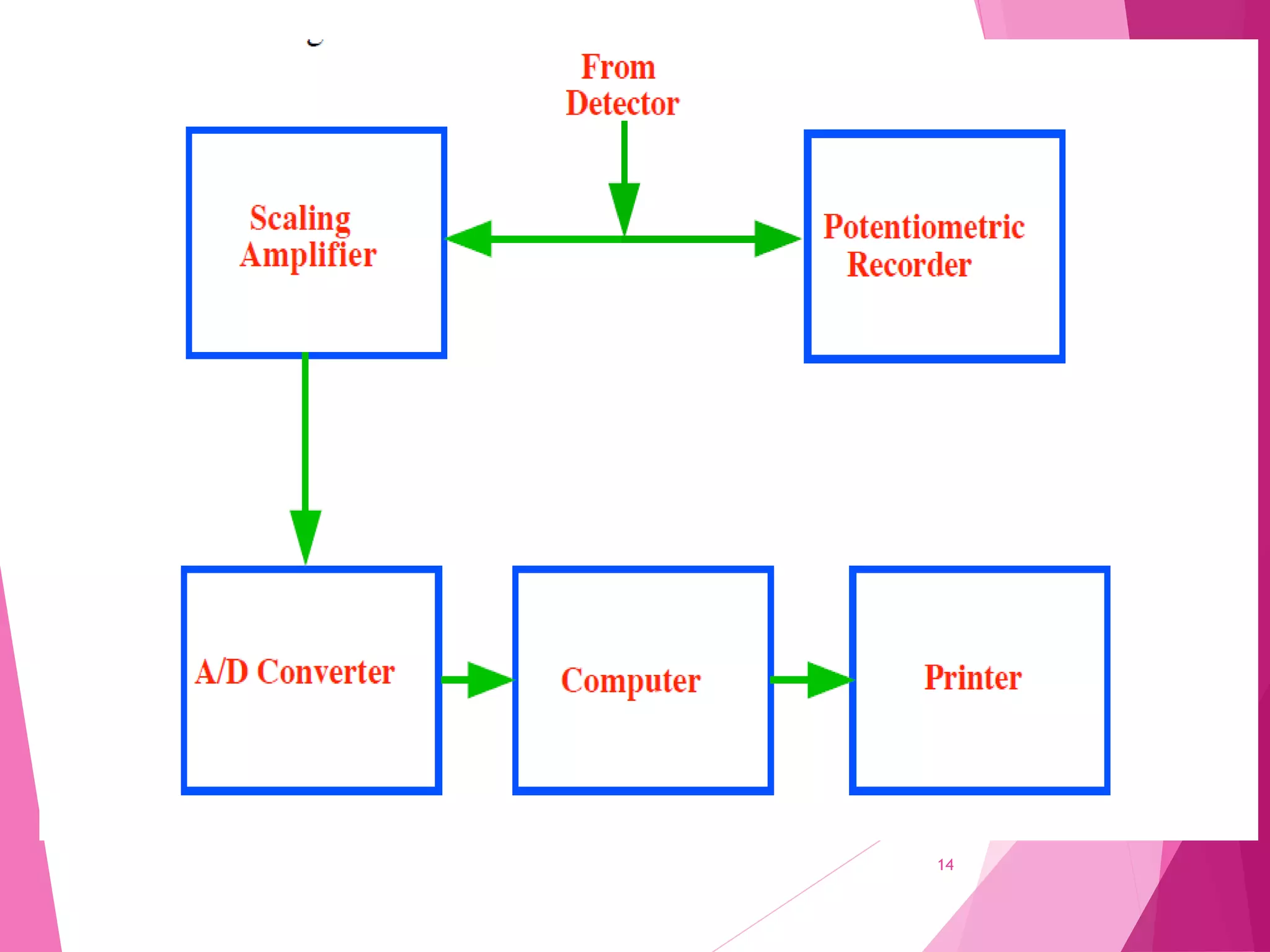
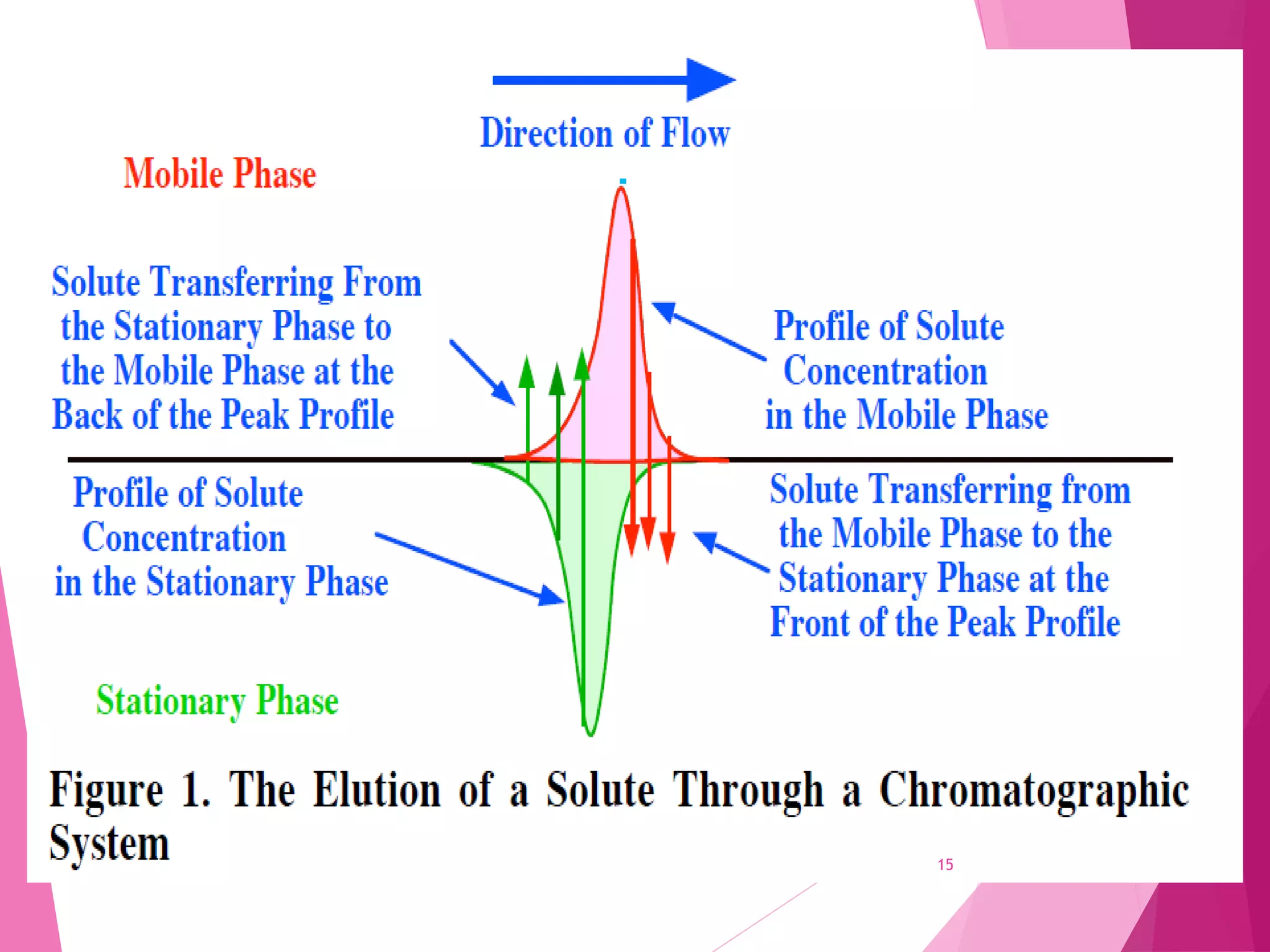
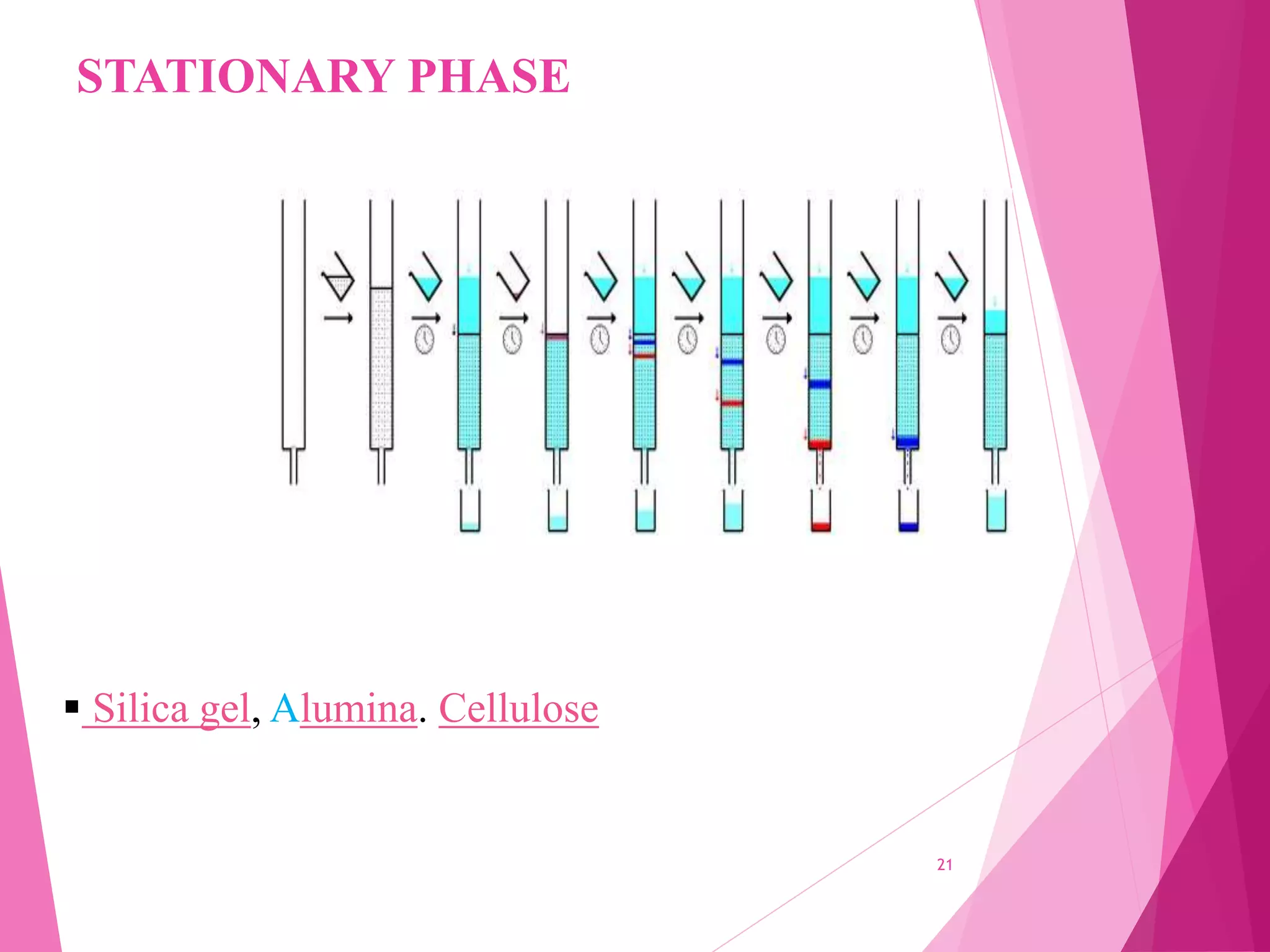
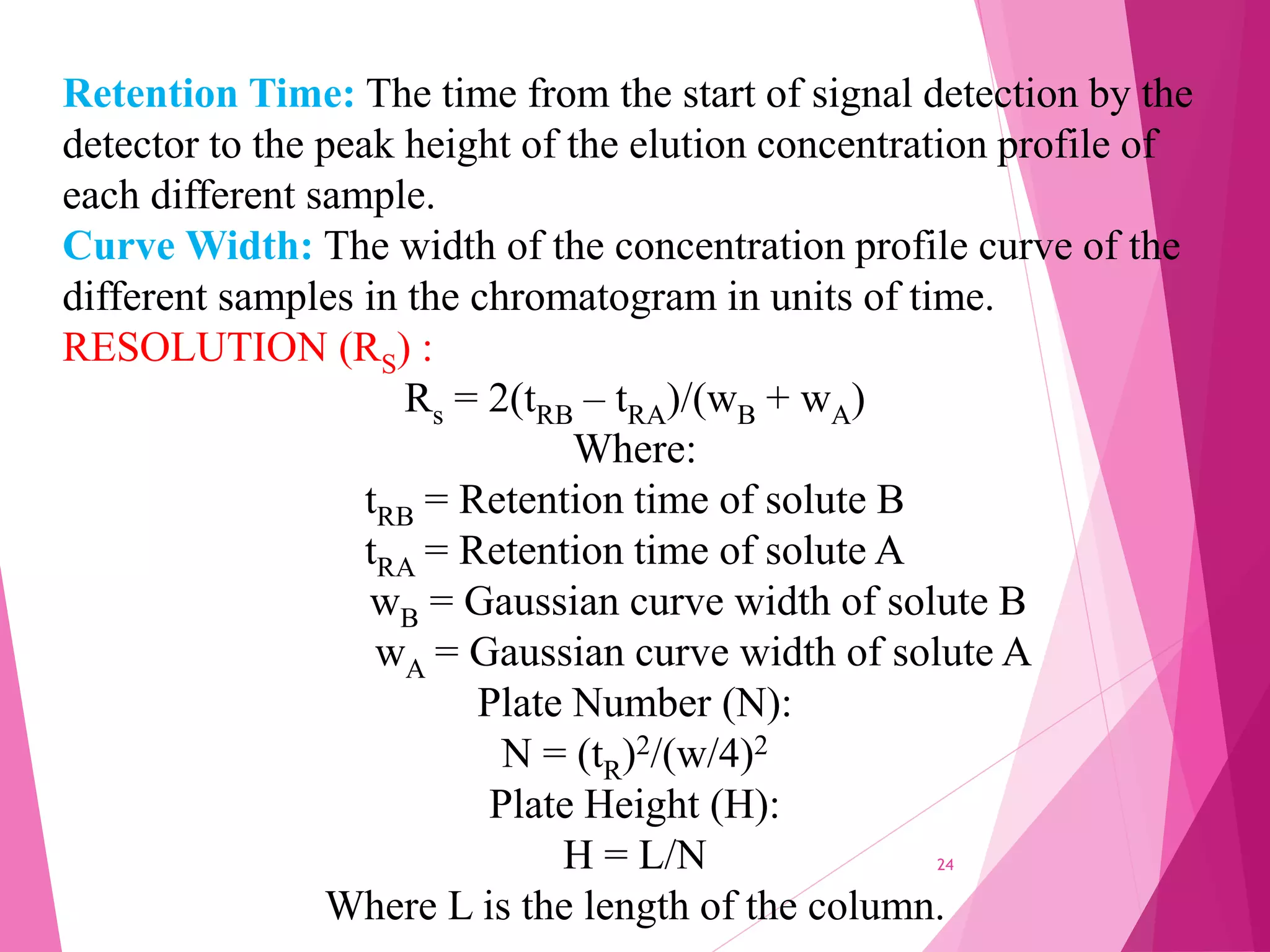
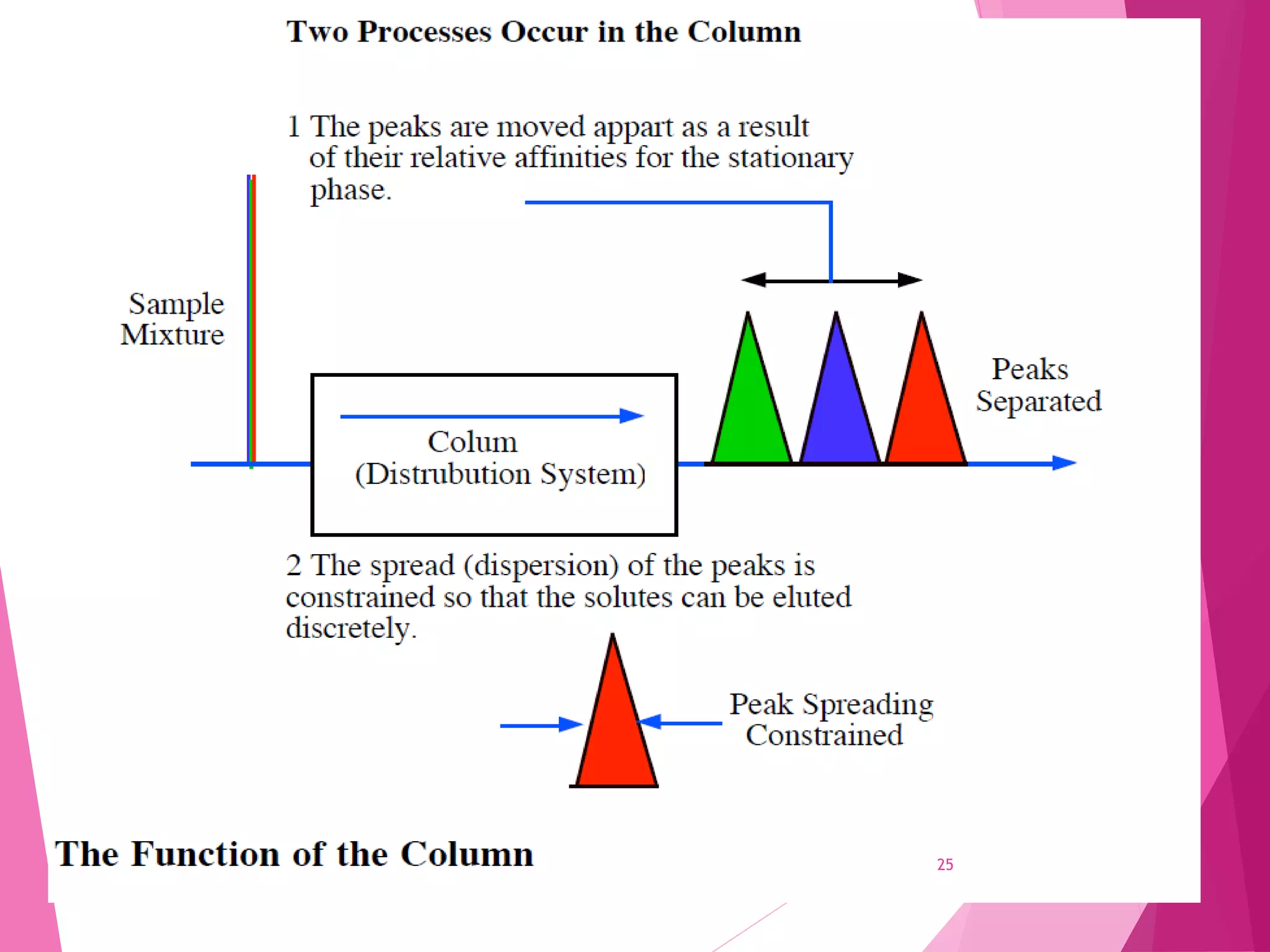
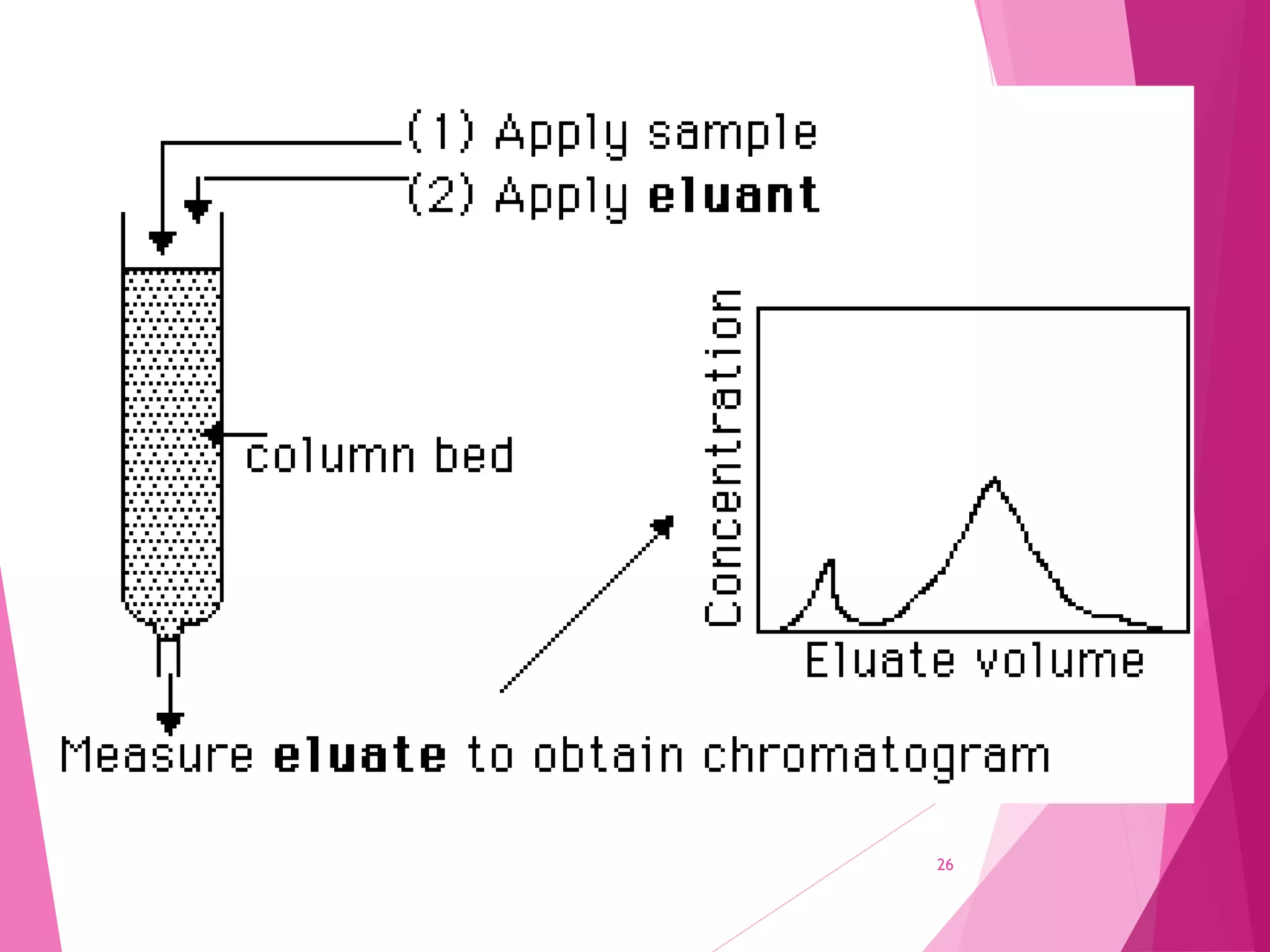
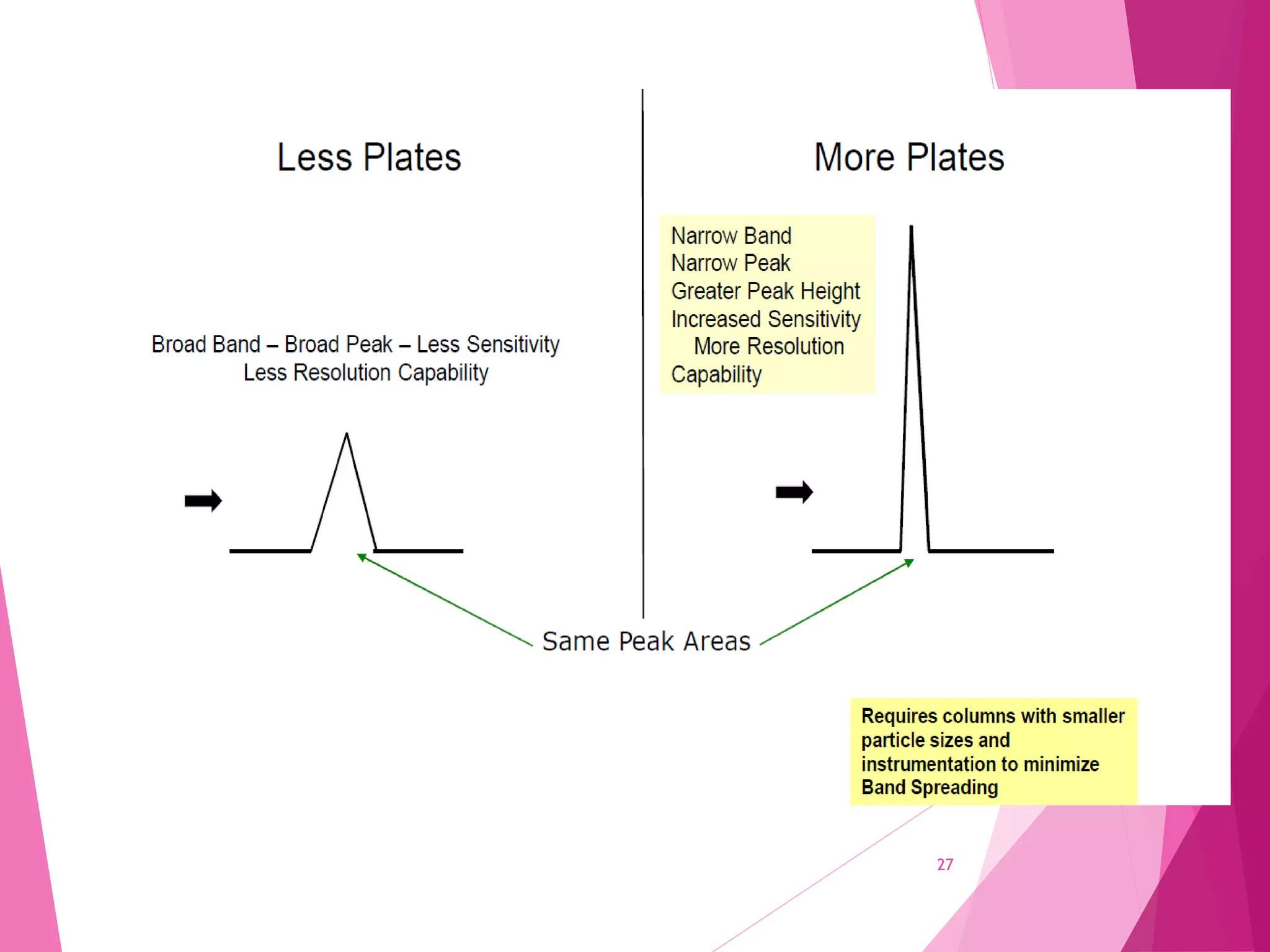
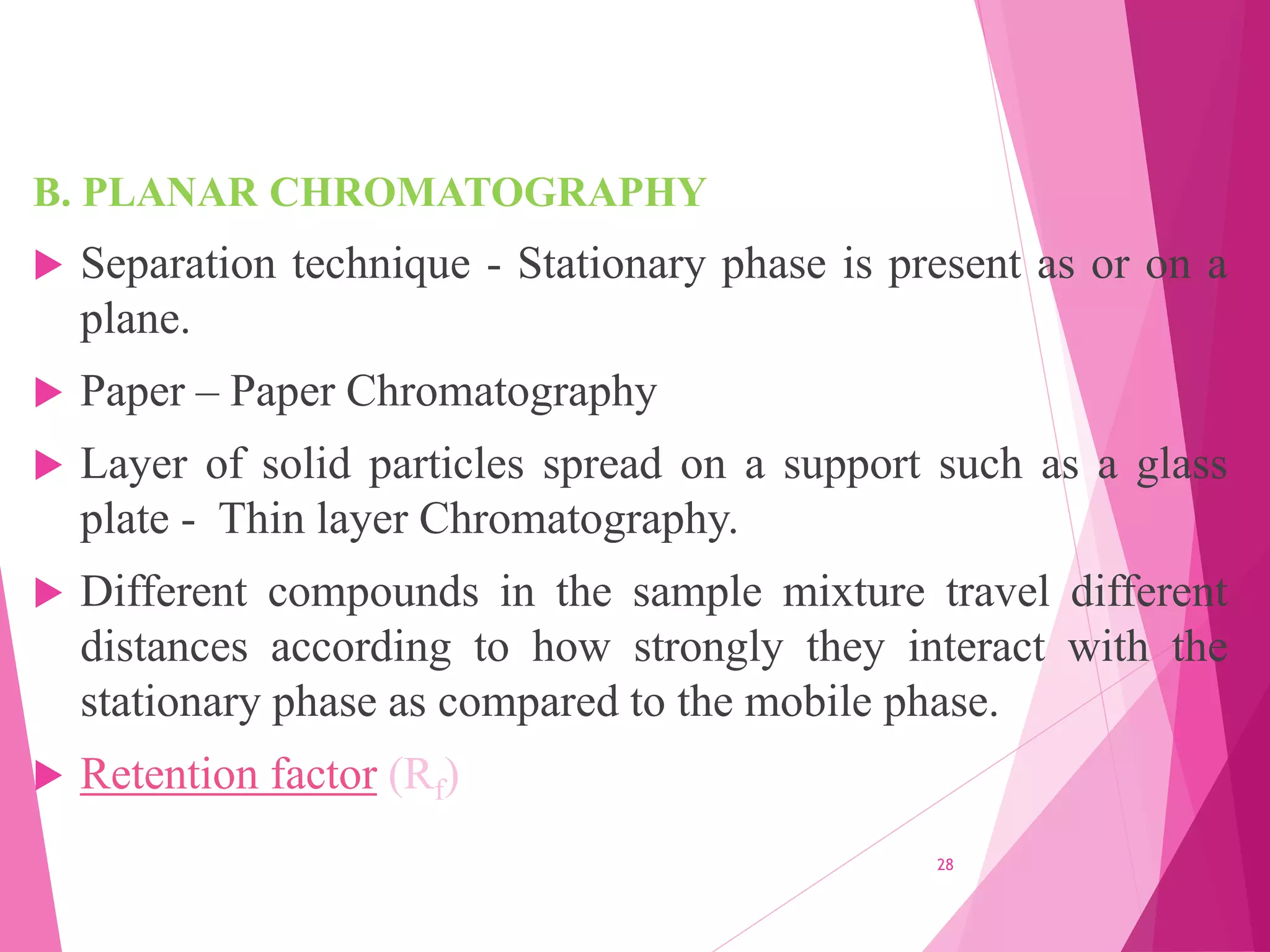
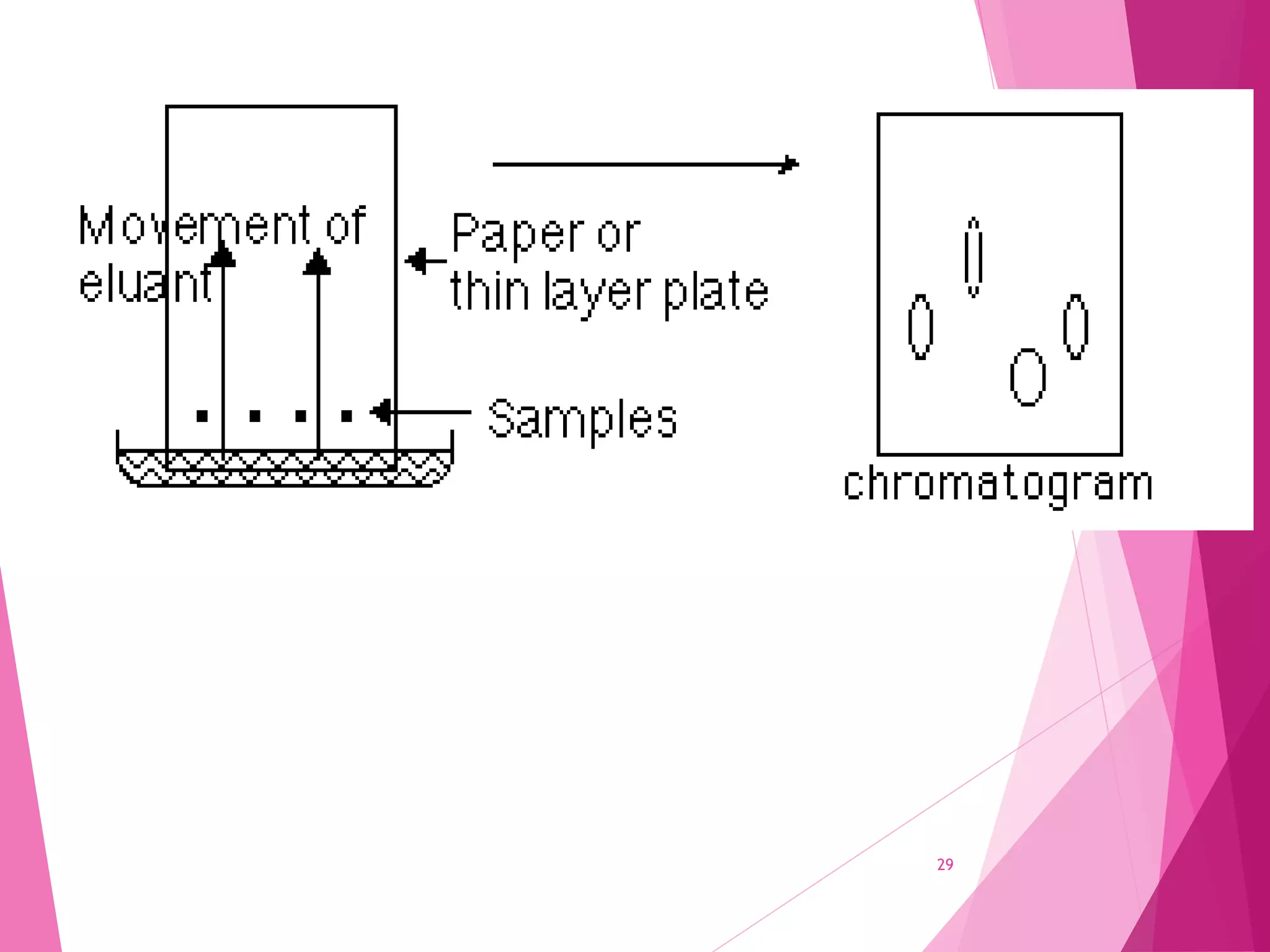
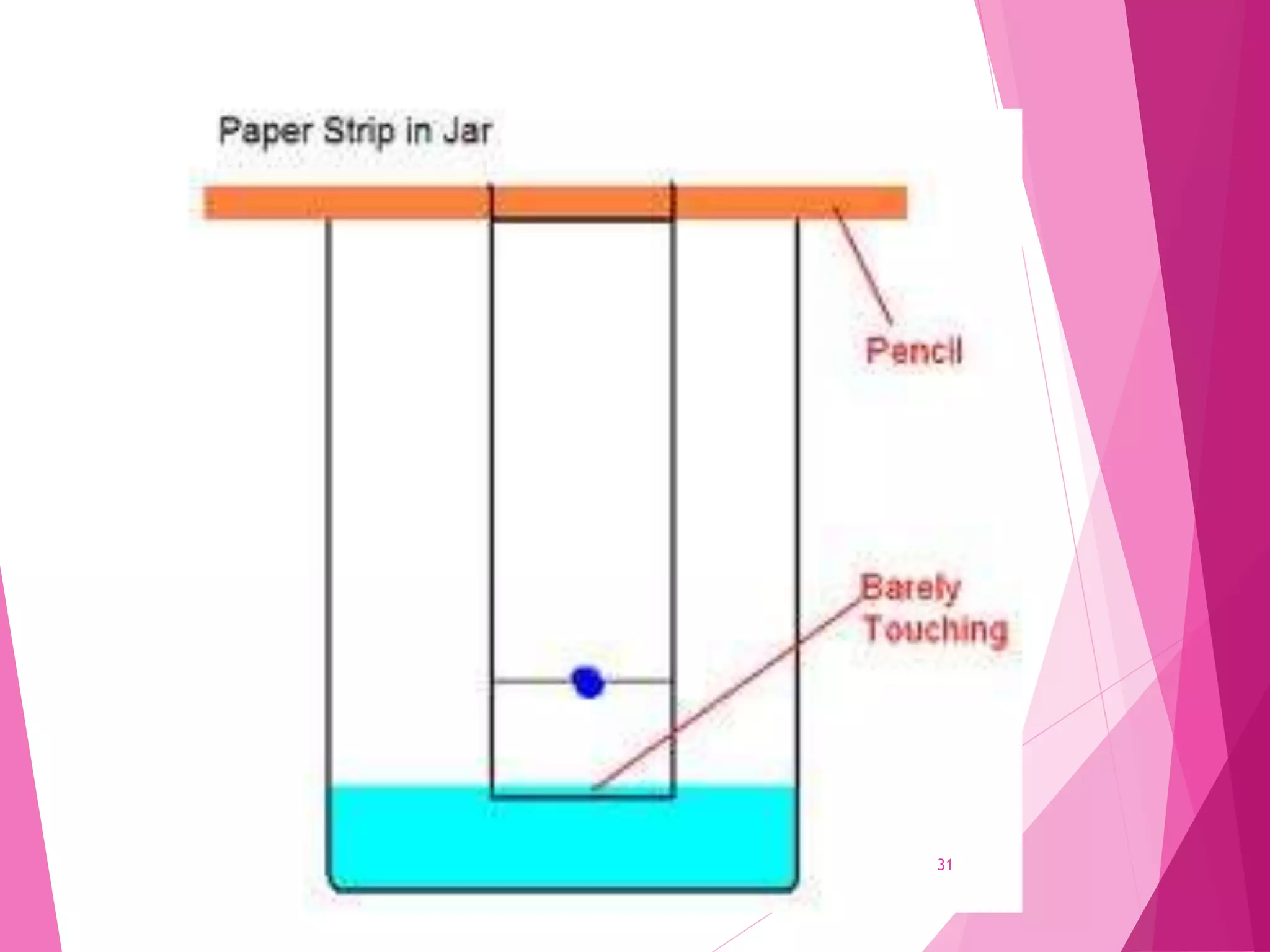
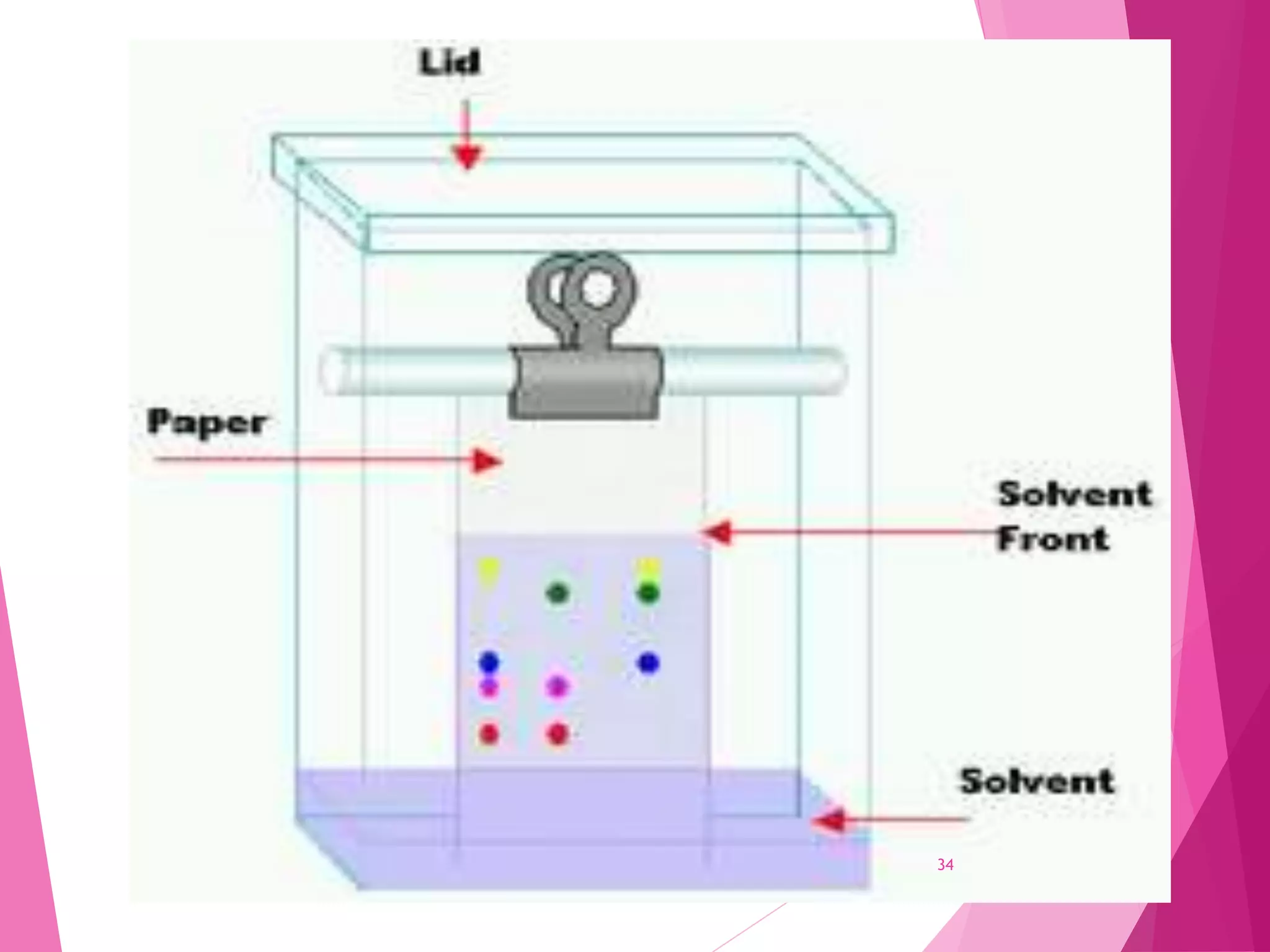
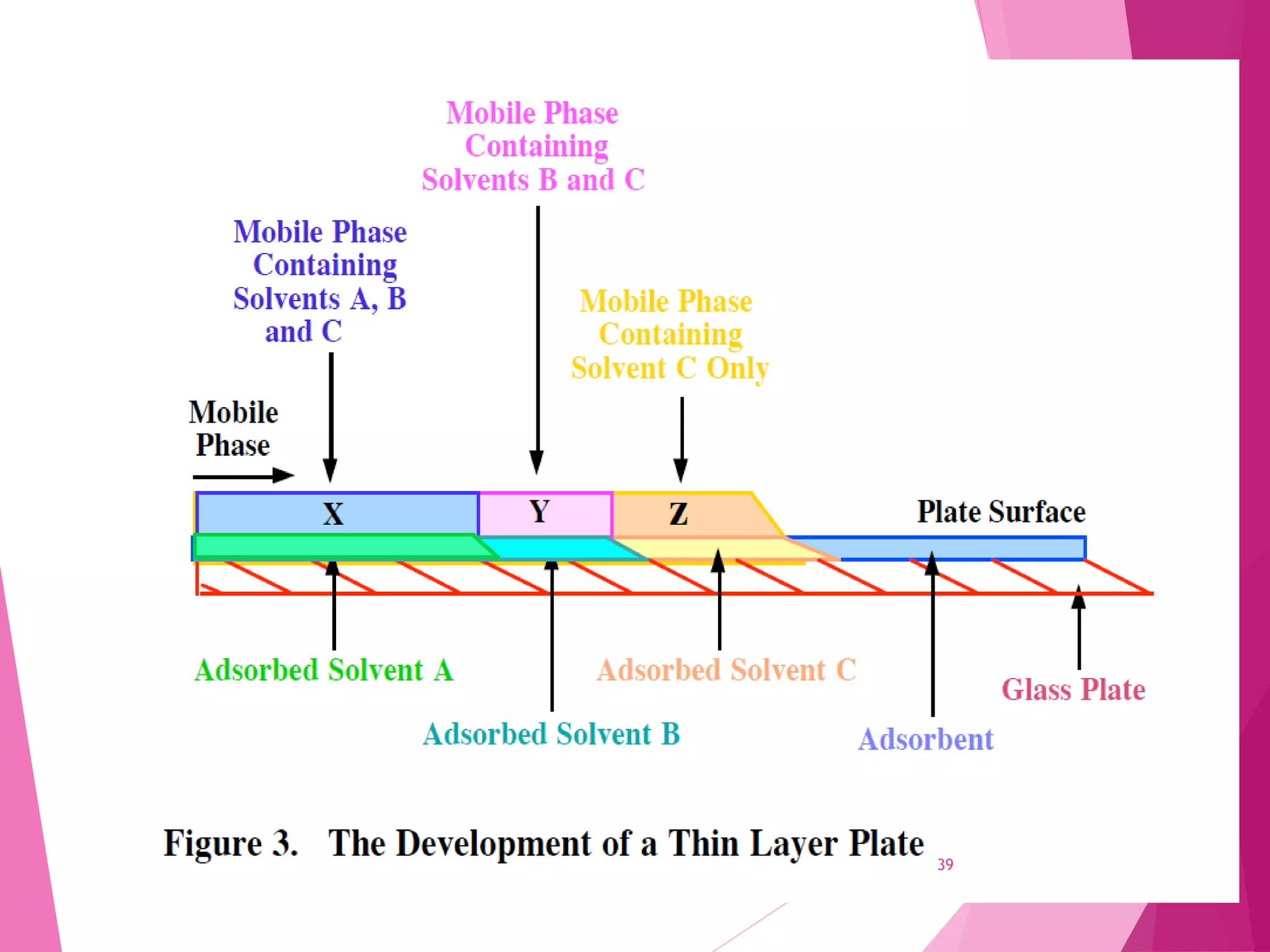
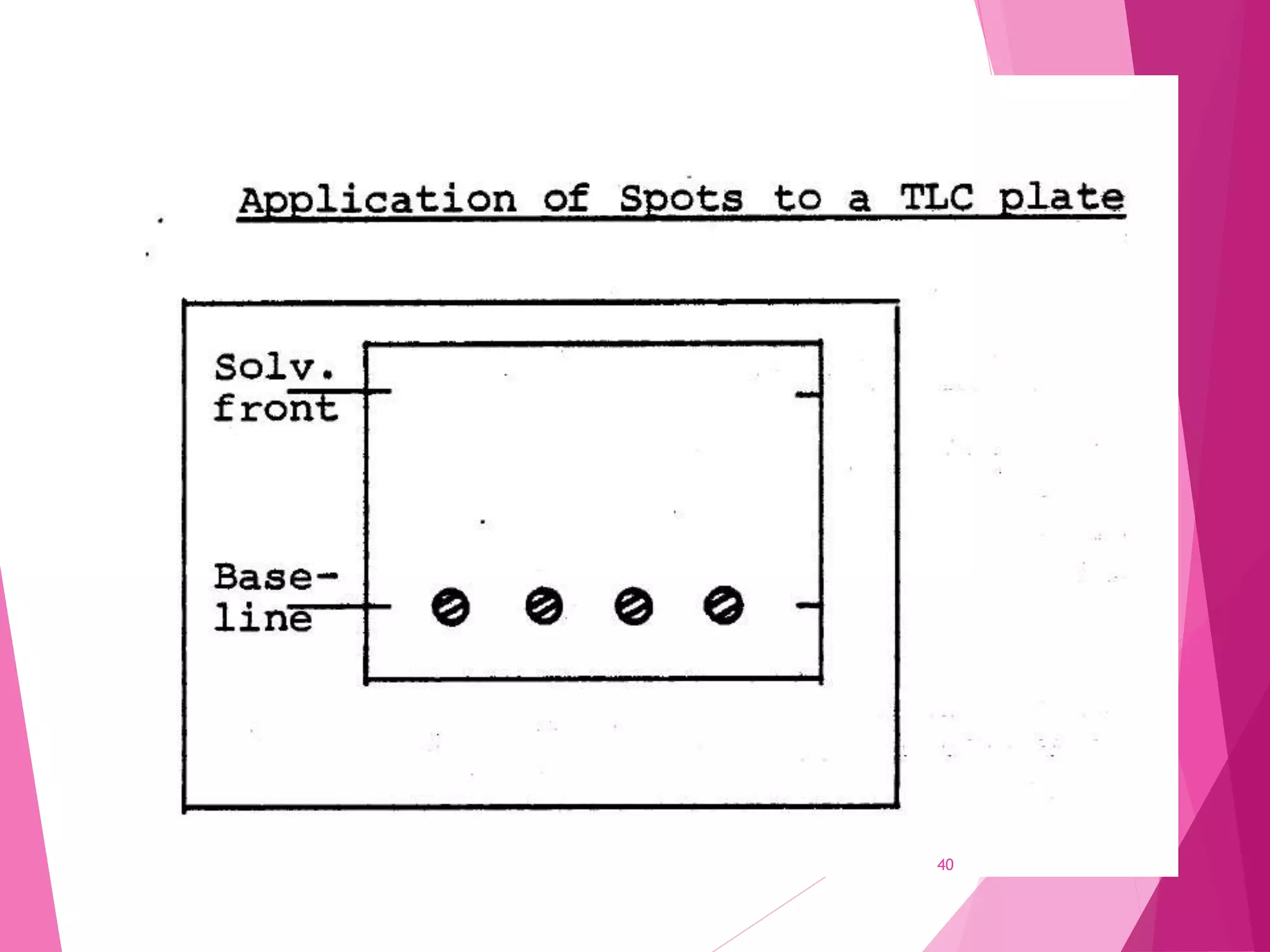
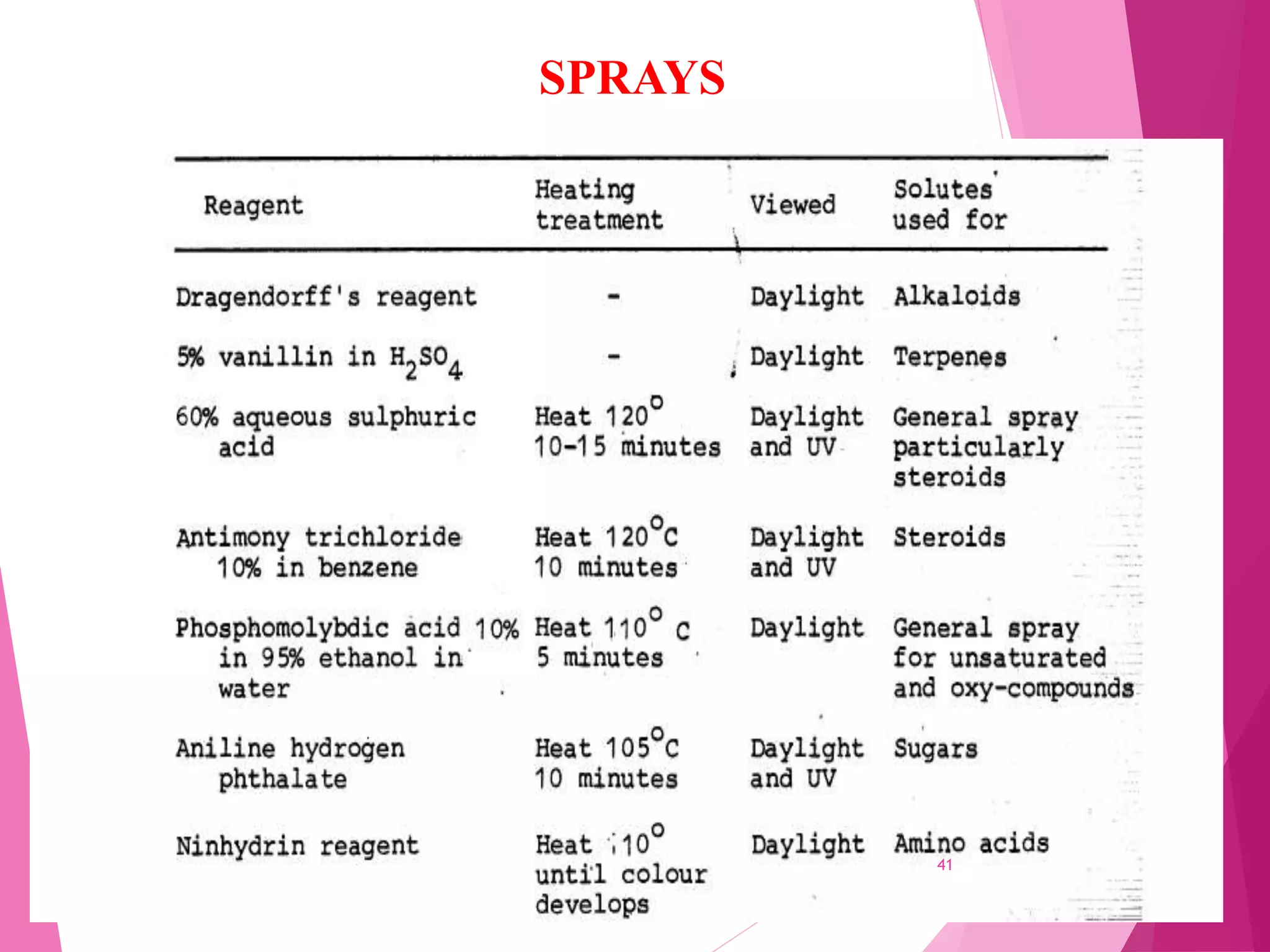
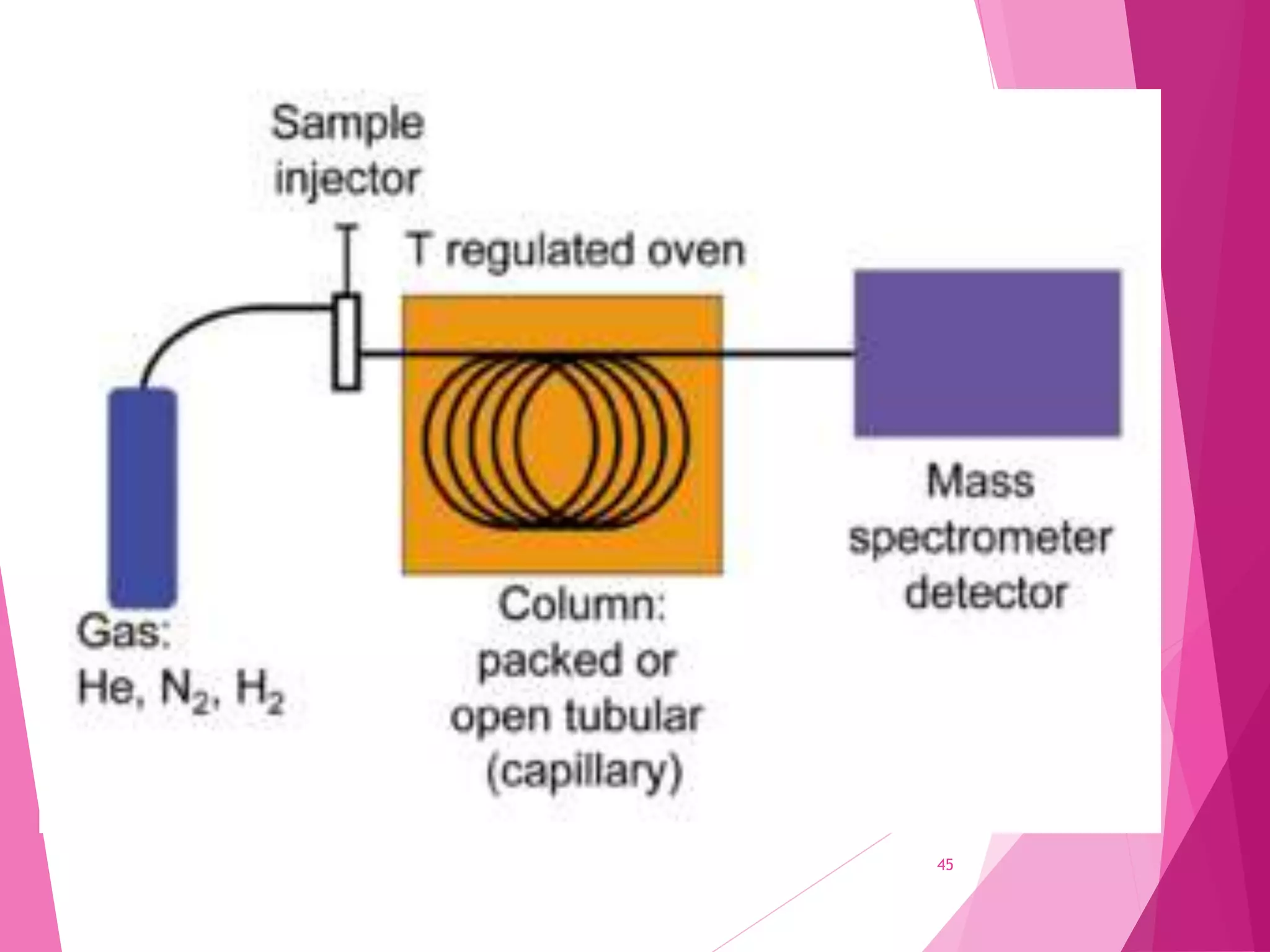
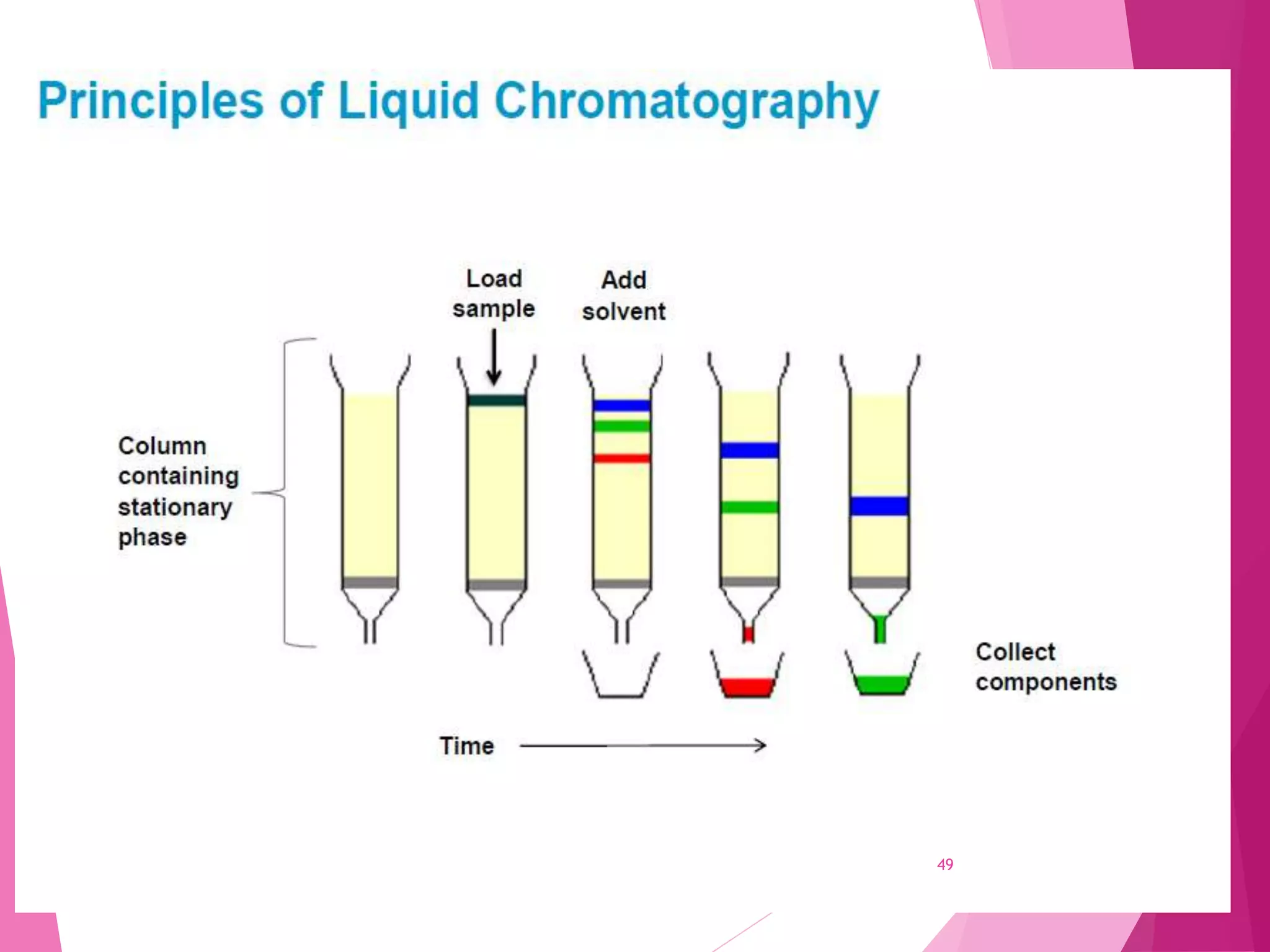
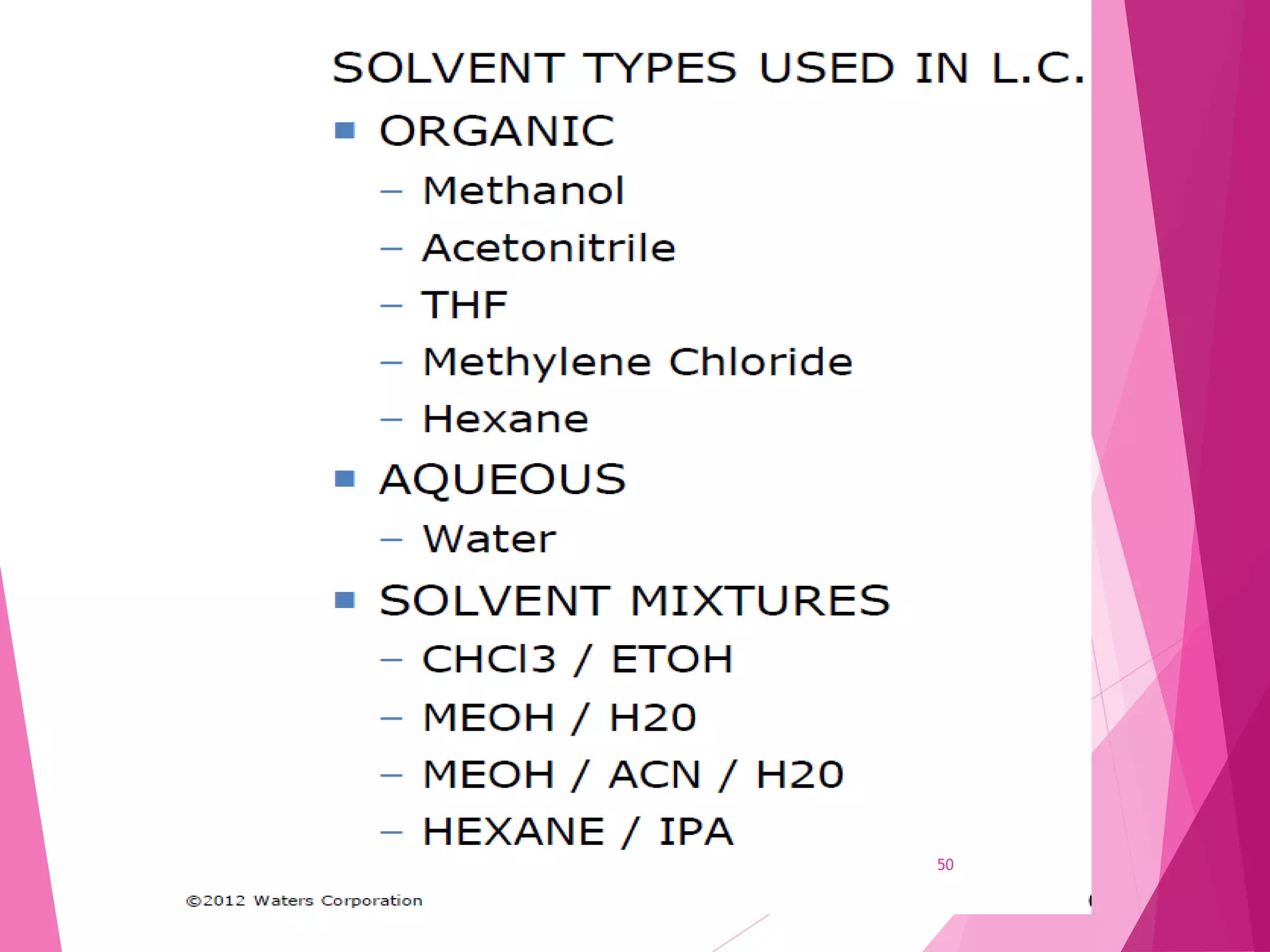
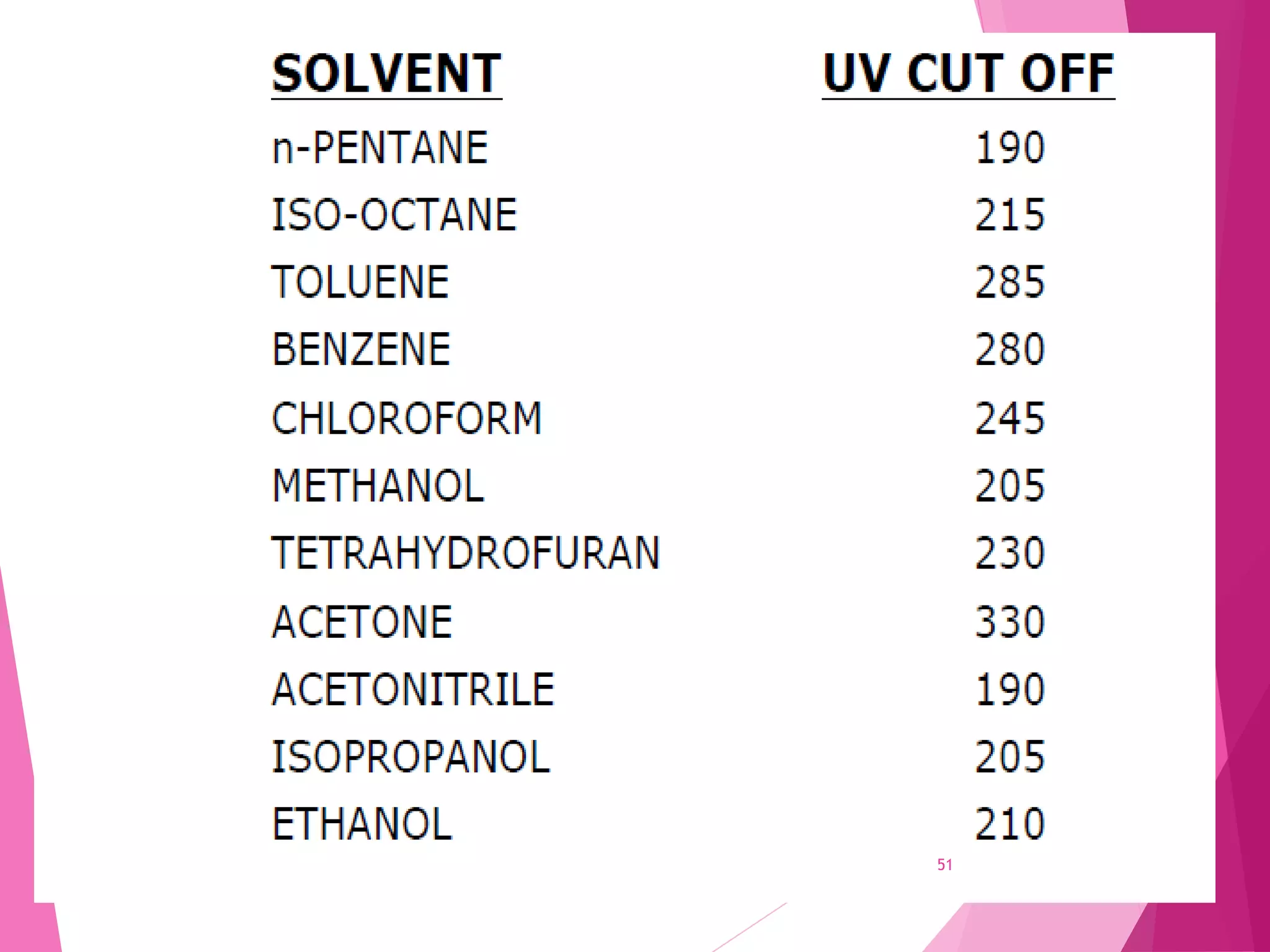
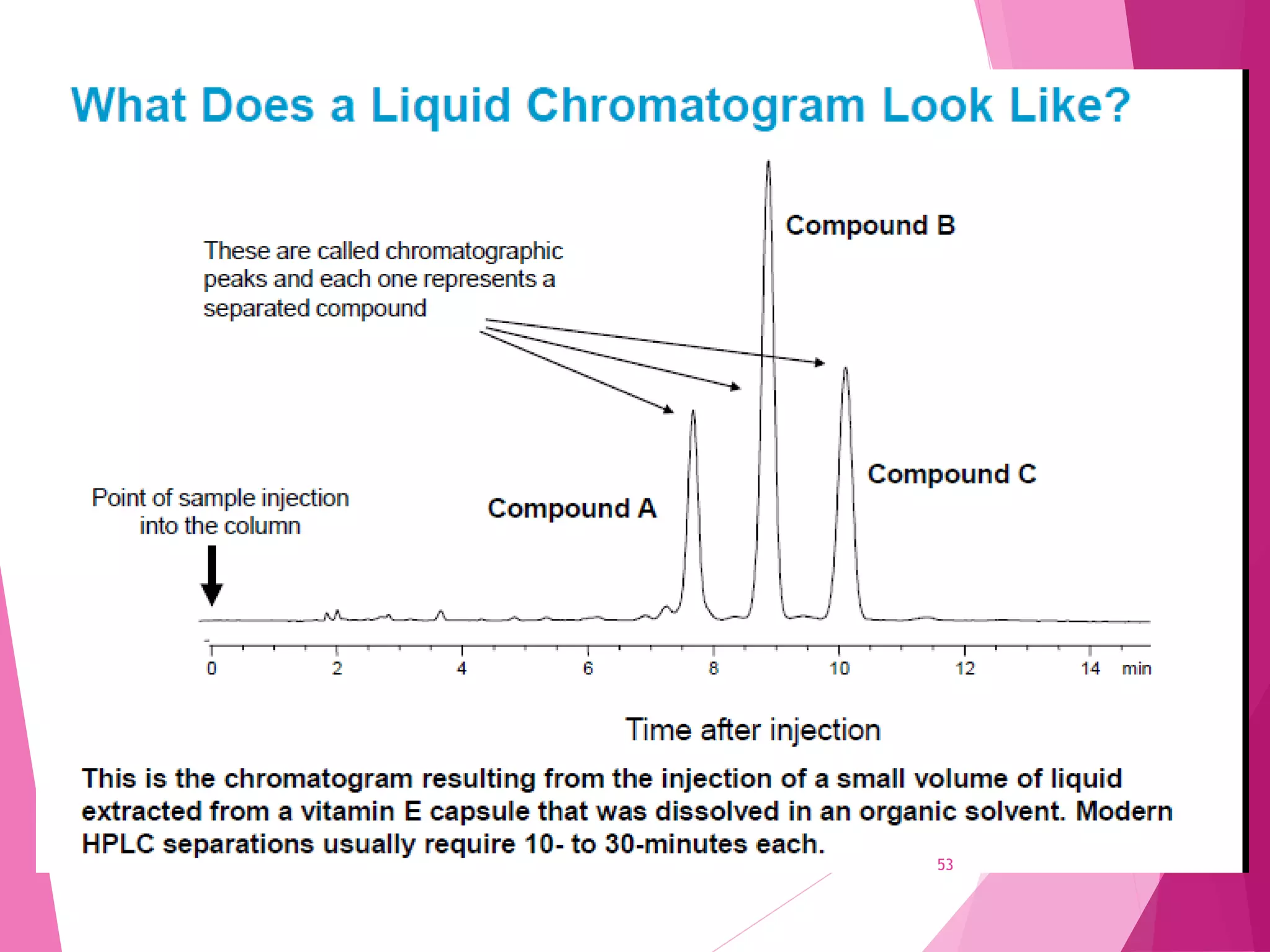
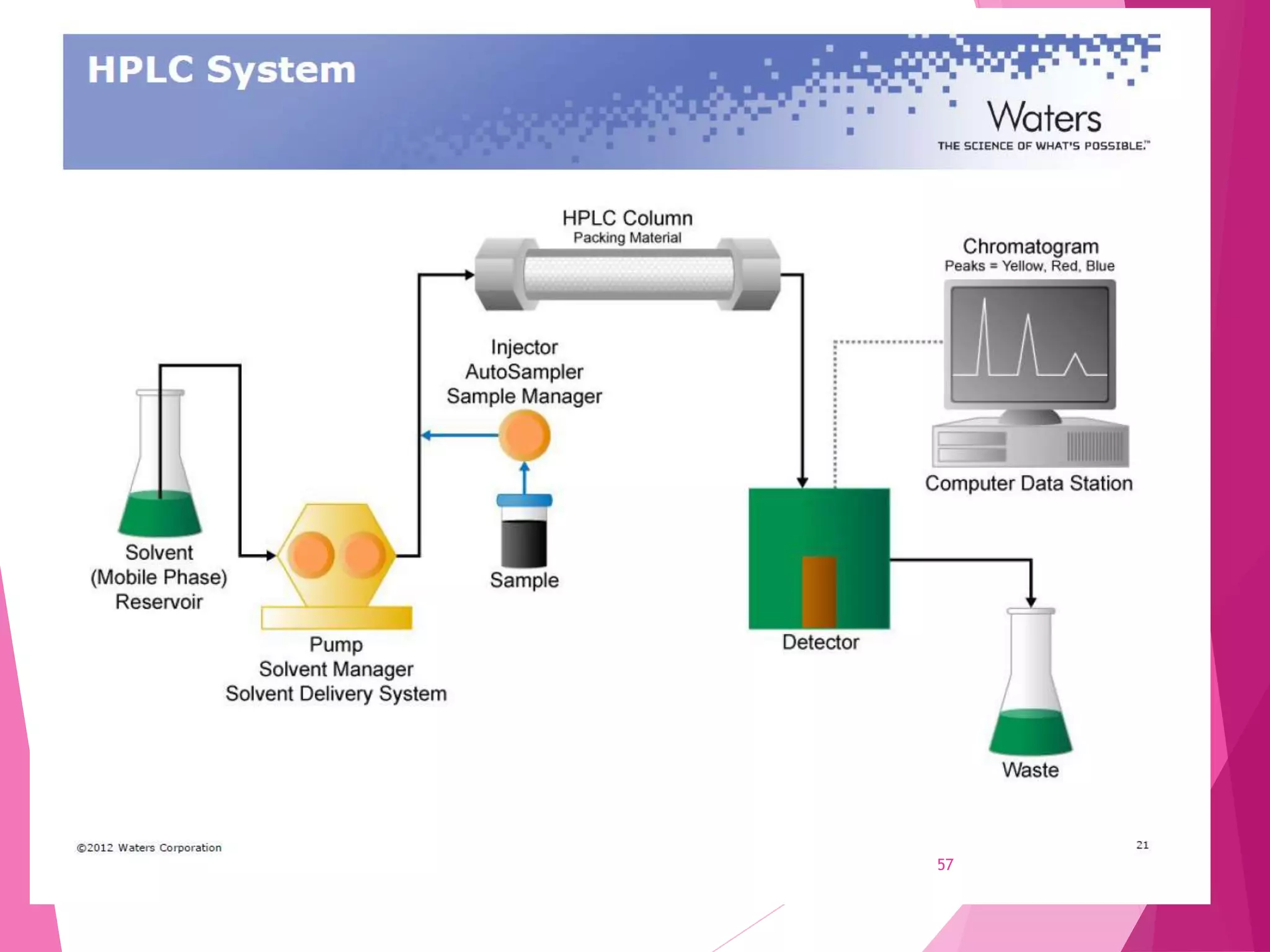
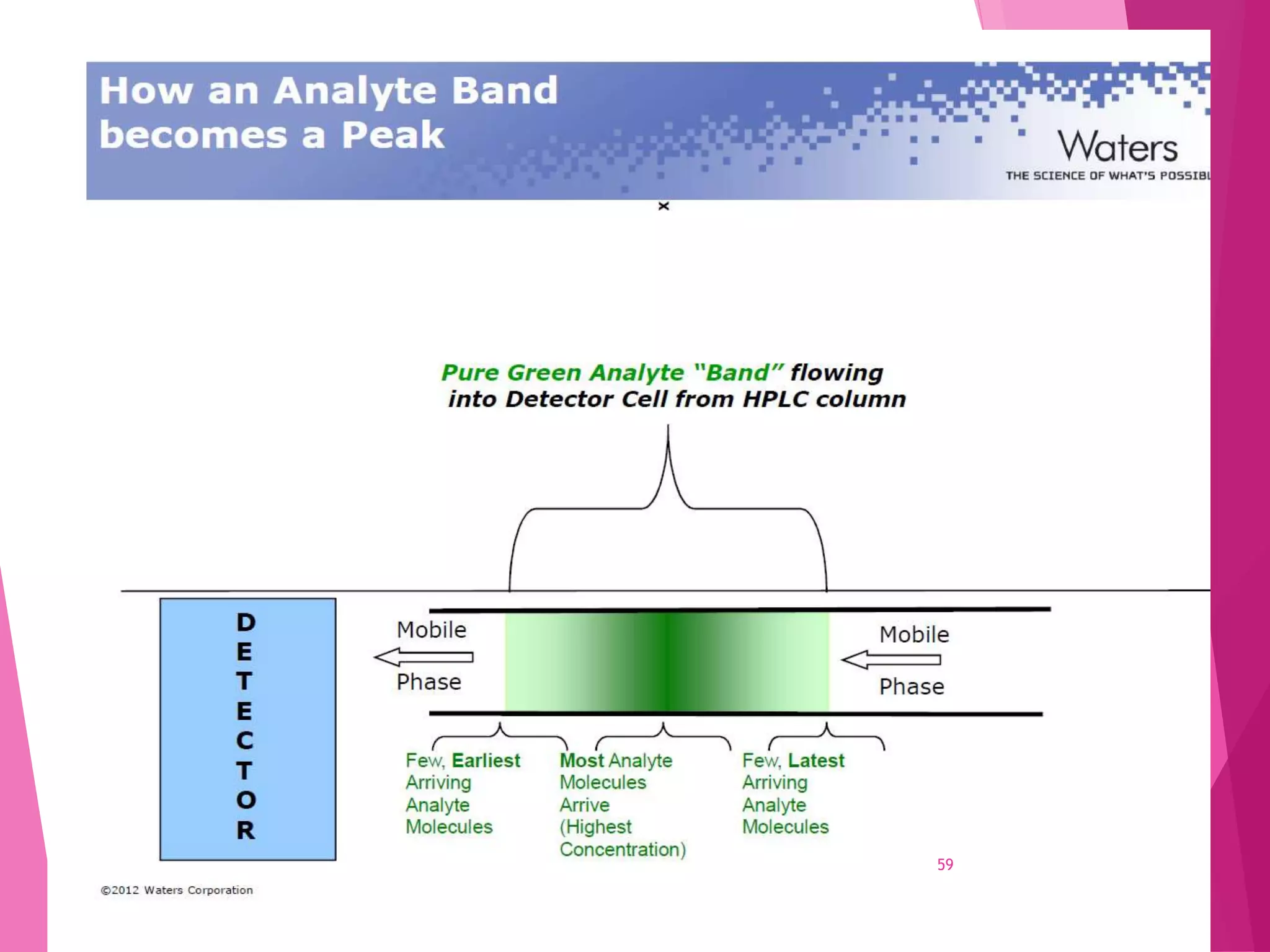
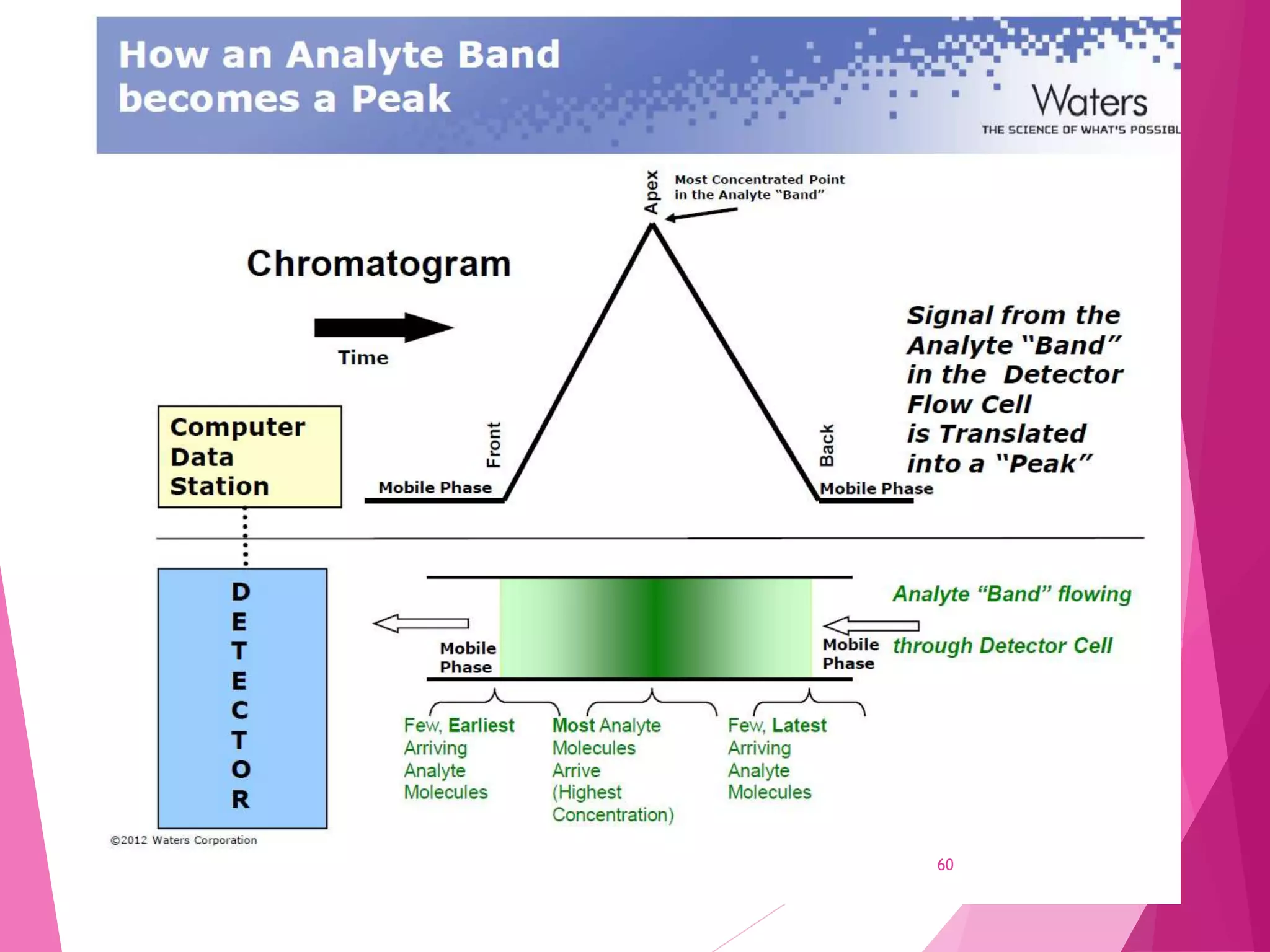
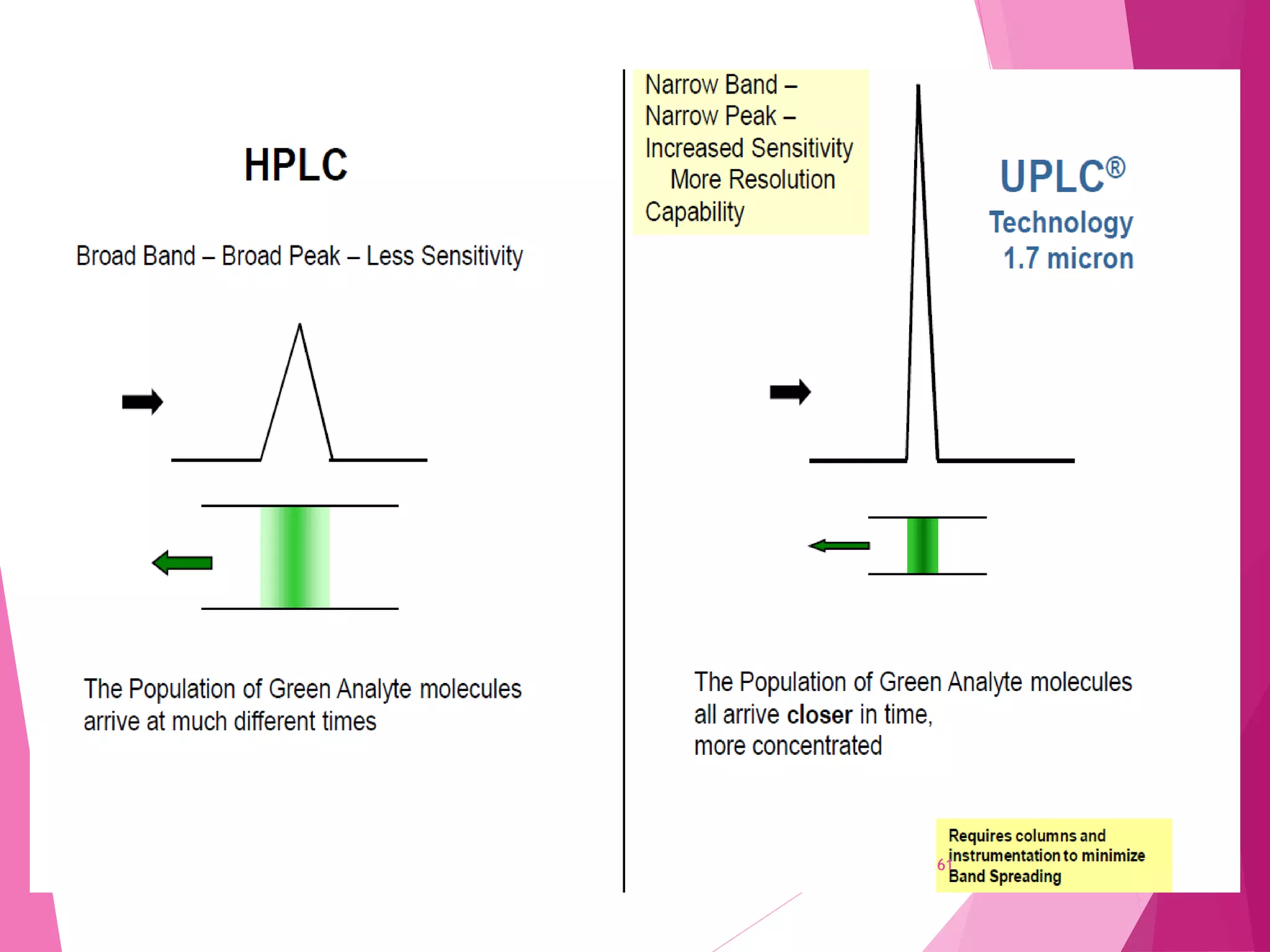
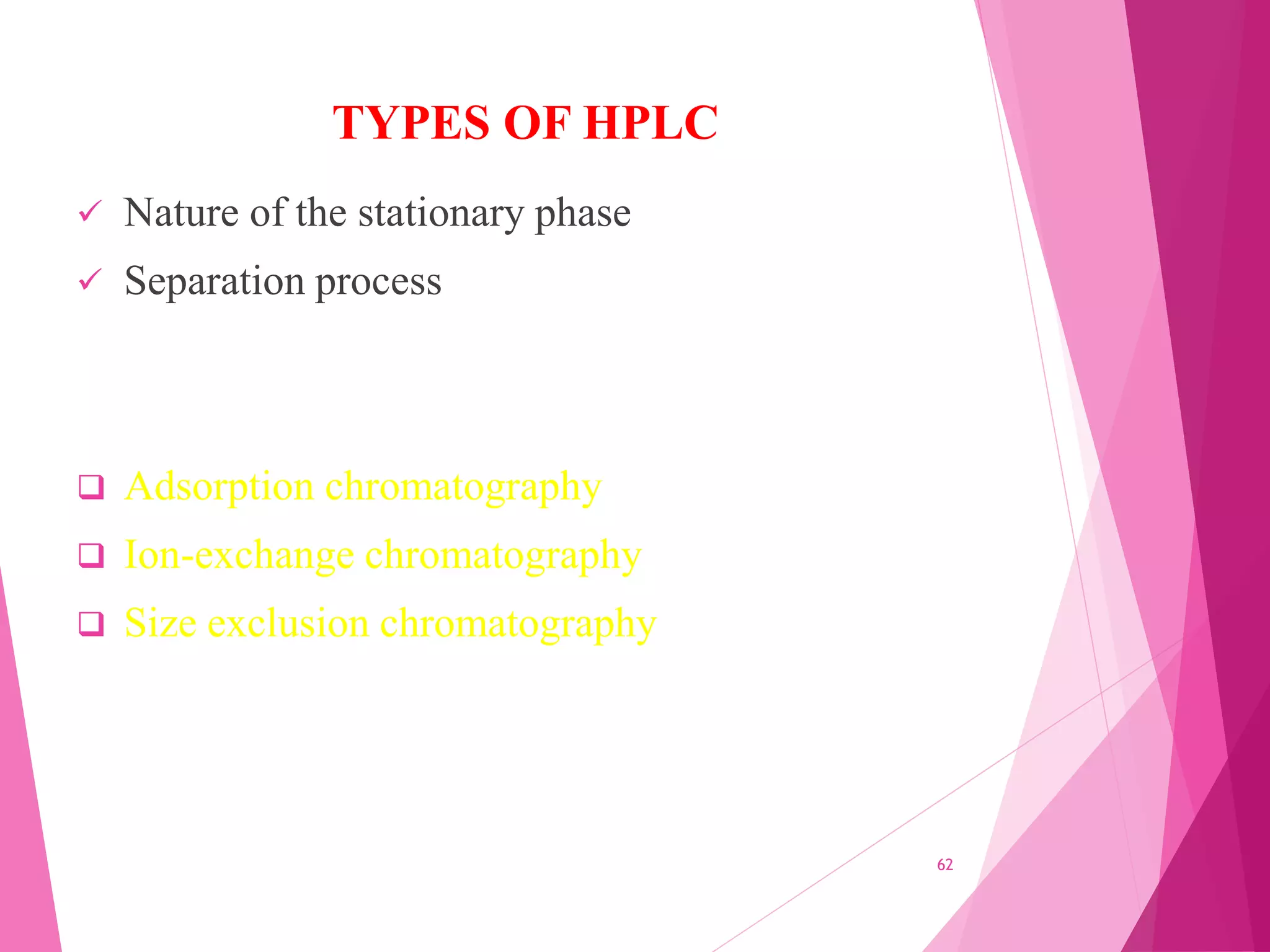
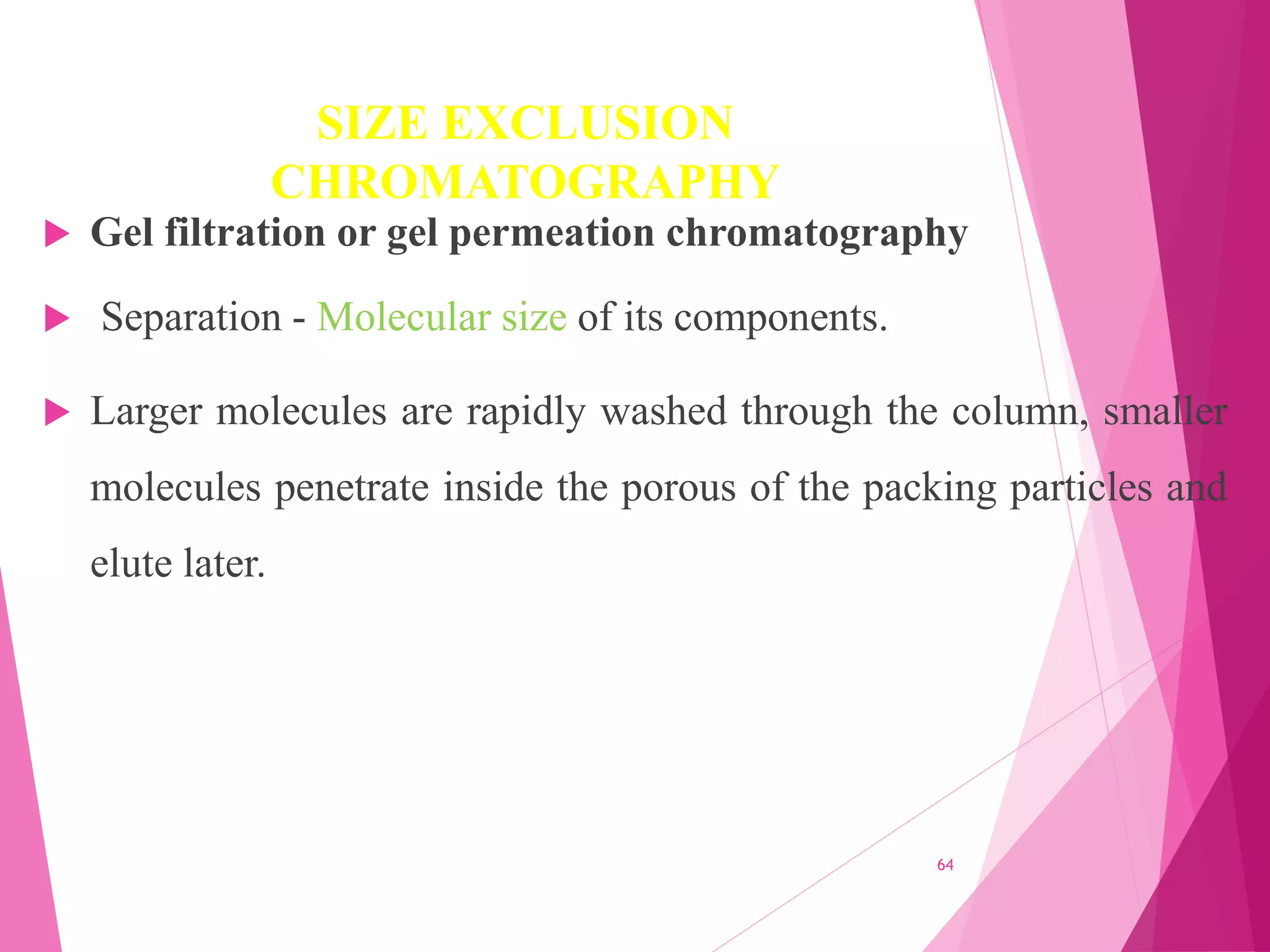
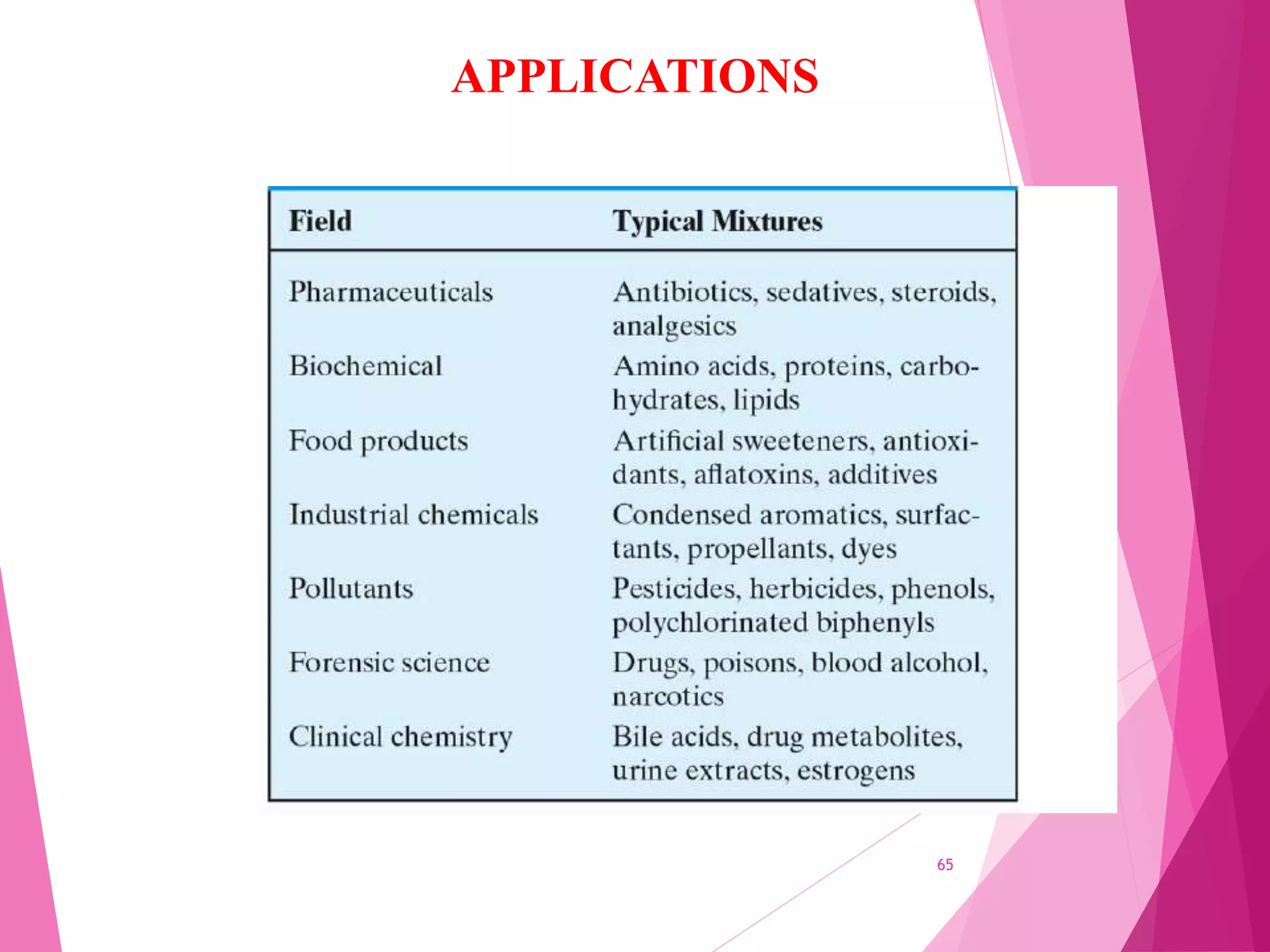
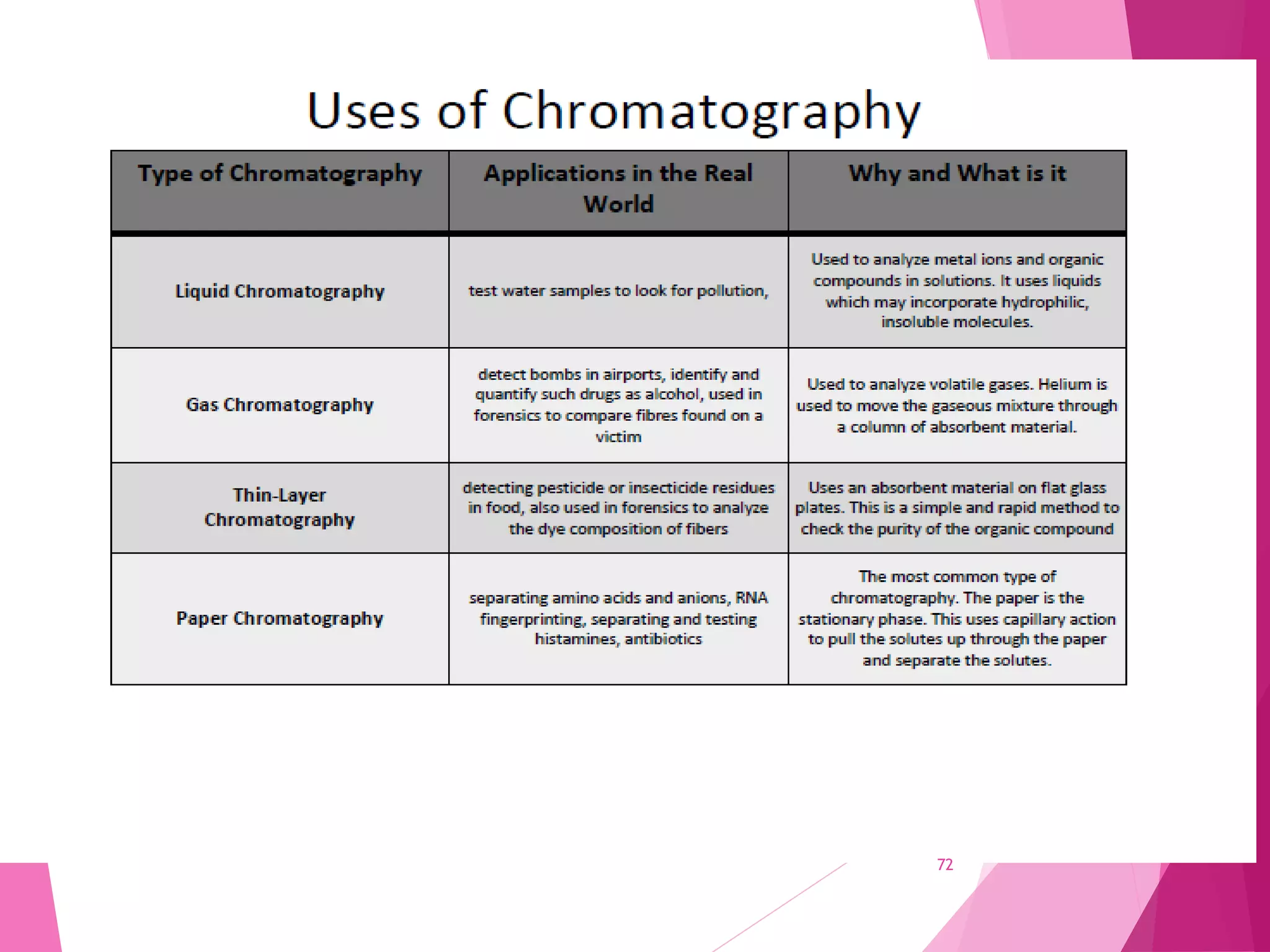
The document provides an extensive overview of chromatography, a laboratory technique for separating mixtures into individual components based on their interactions with a mobile and stationary phase. It discusses various chromatography methods such as gas chromatography, liquid chromatography, and thin layer chromatography, highlighting principles, terms, techniques, applications, and factors affecting separation. Additionally, it includes details on the detection methods and the importance of parameters like retention time and resolution in analyzing samples.