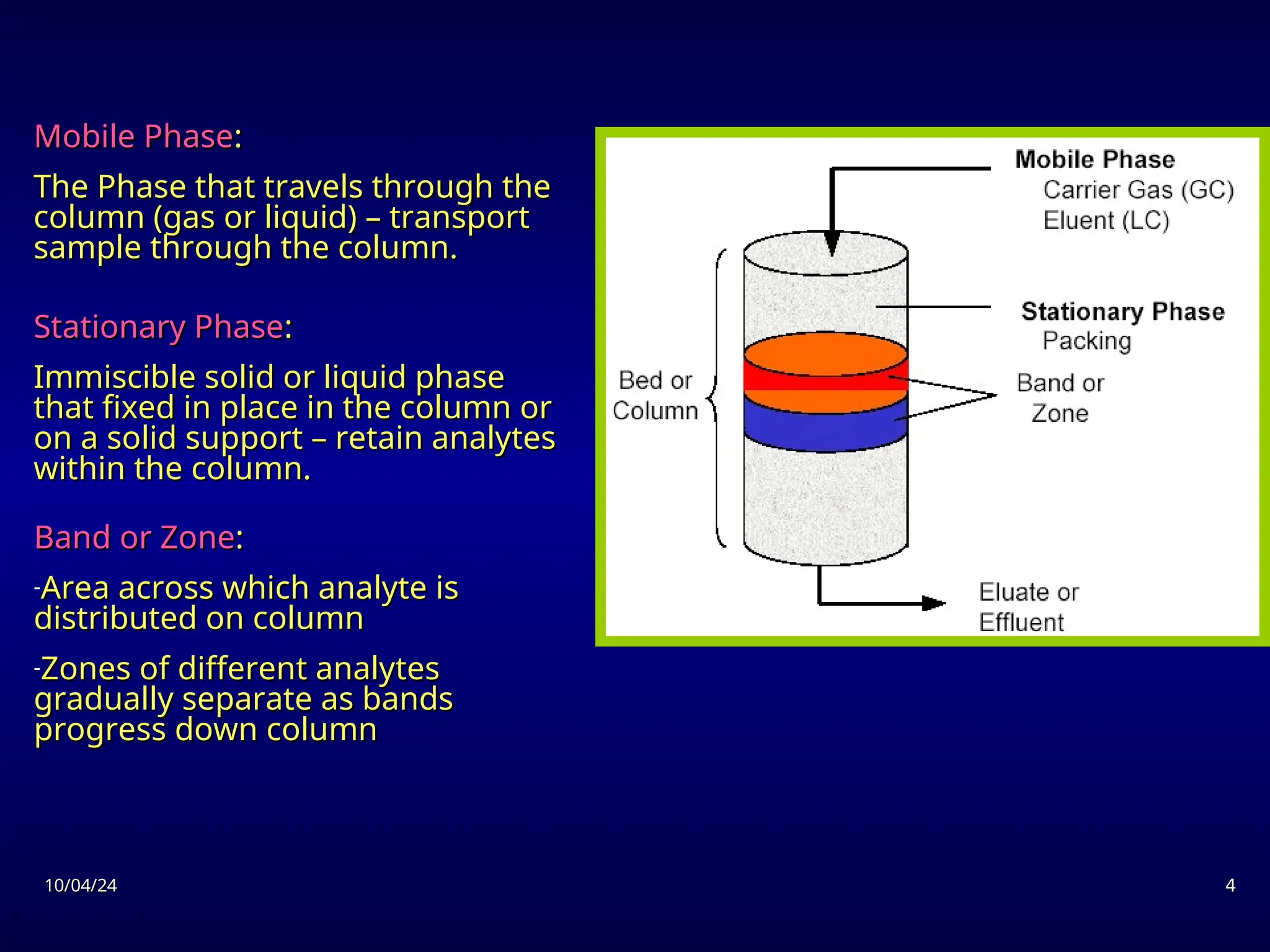
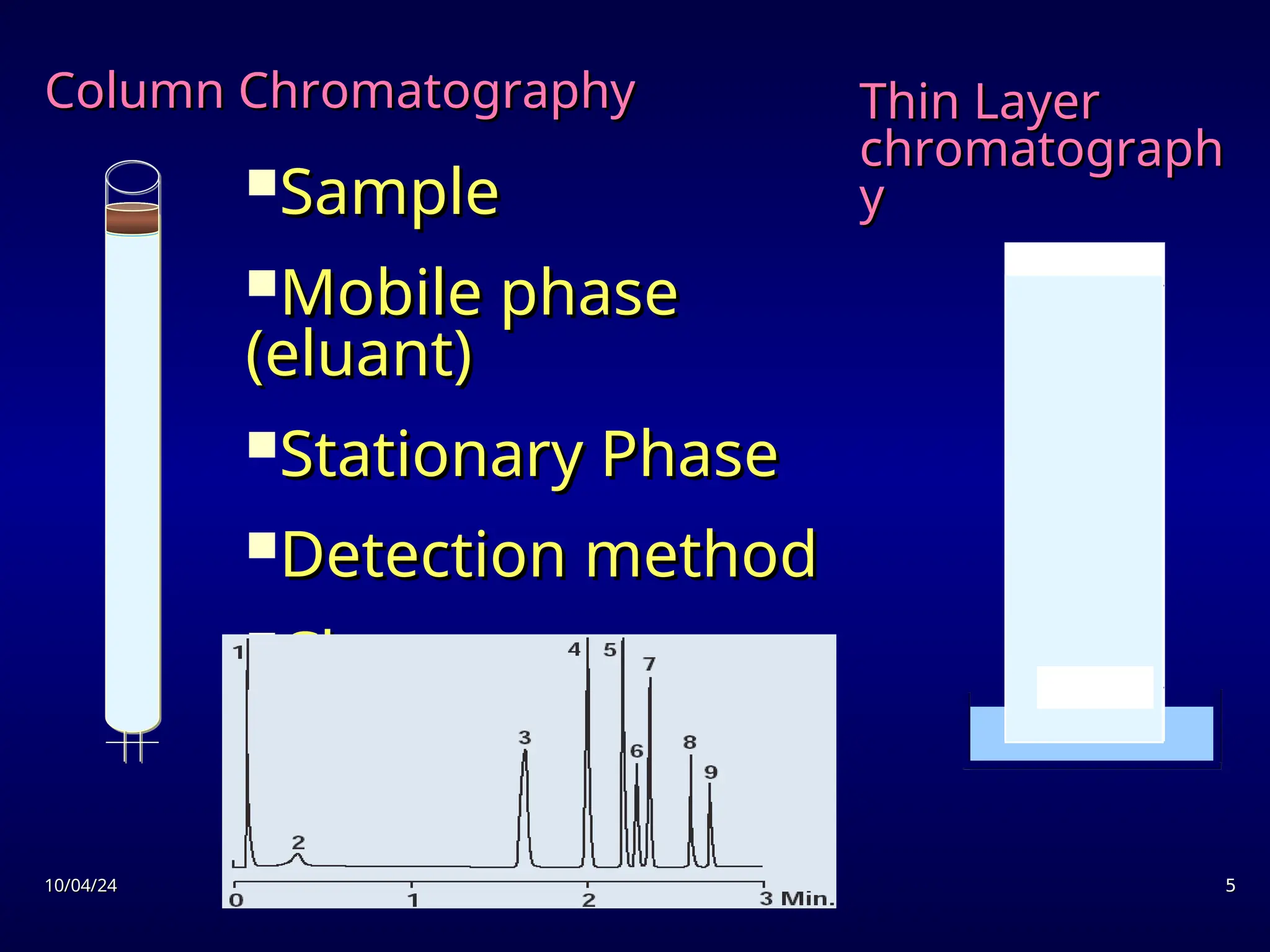
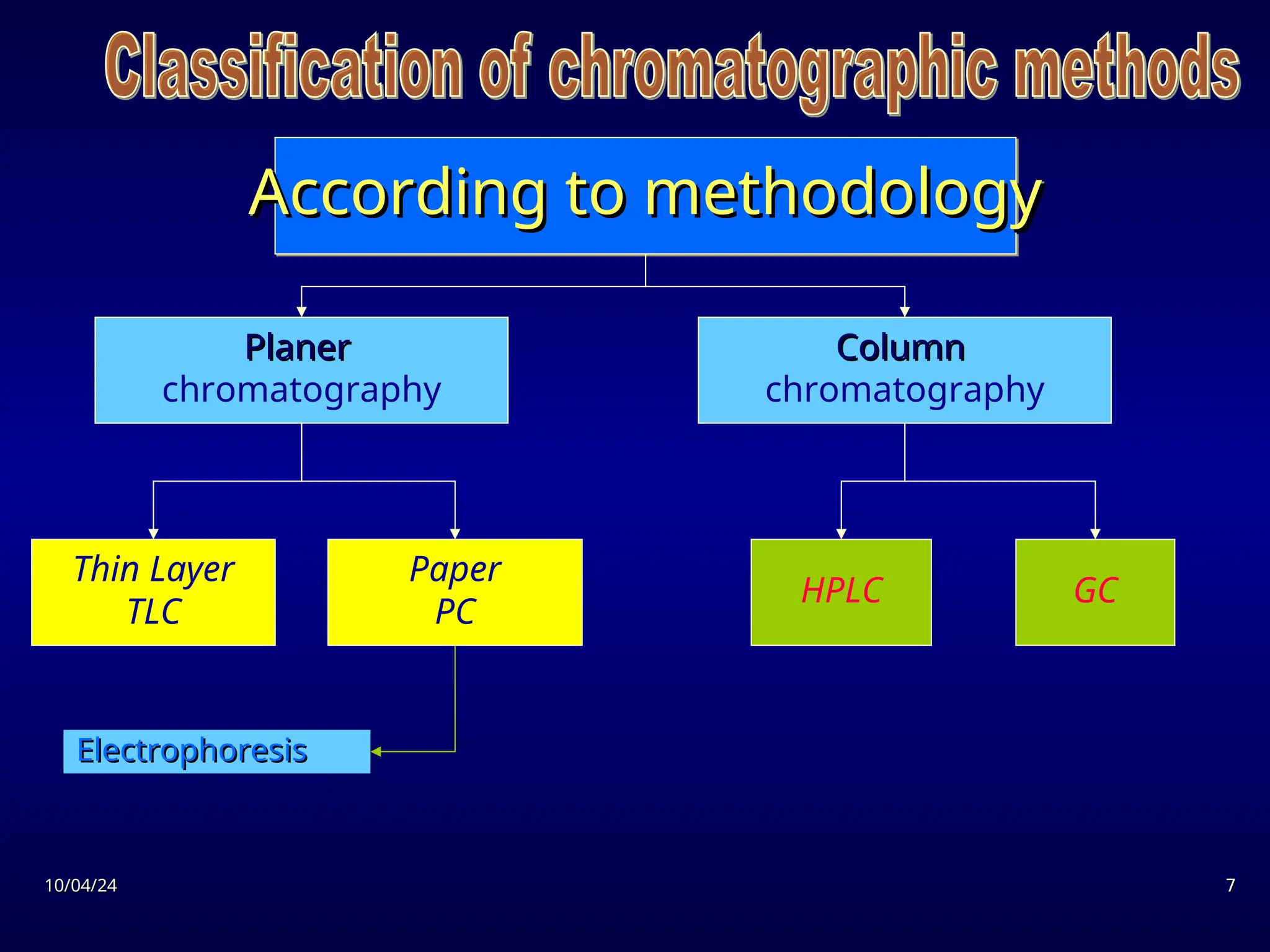
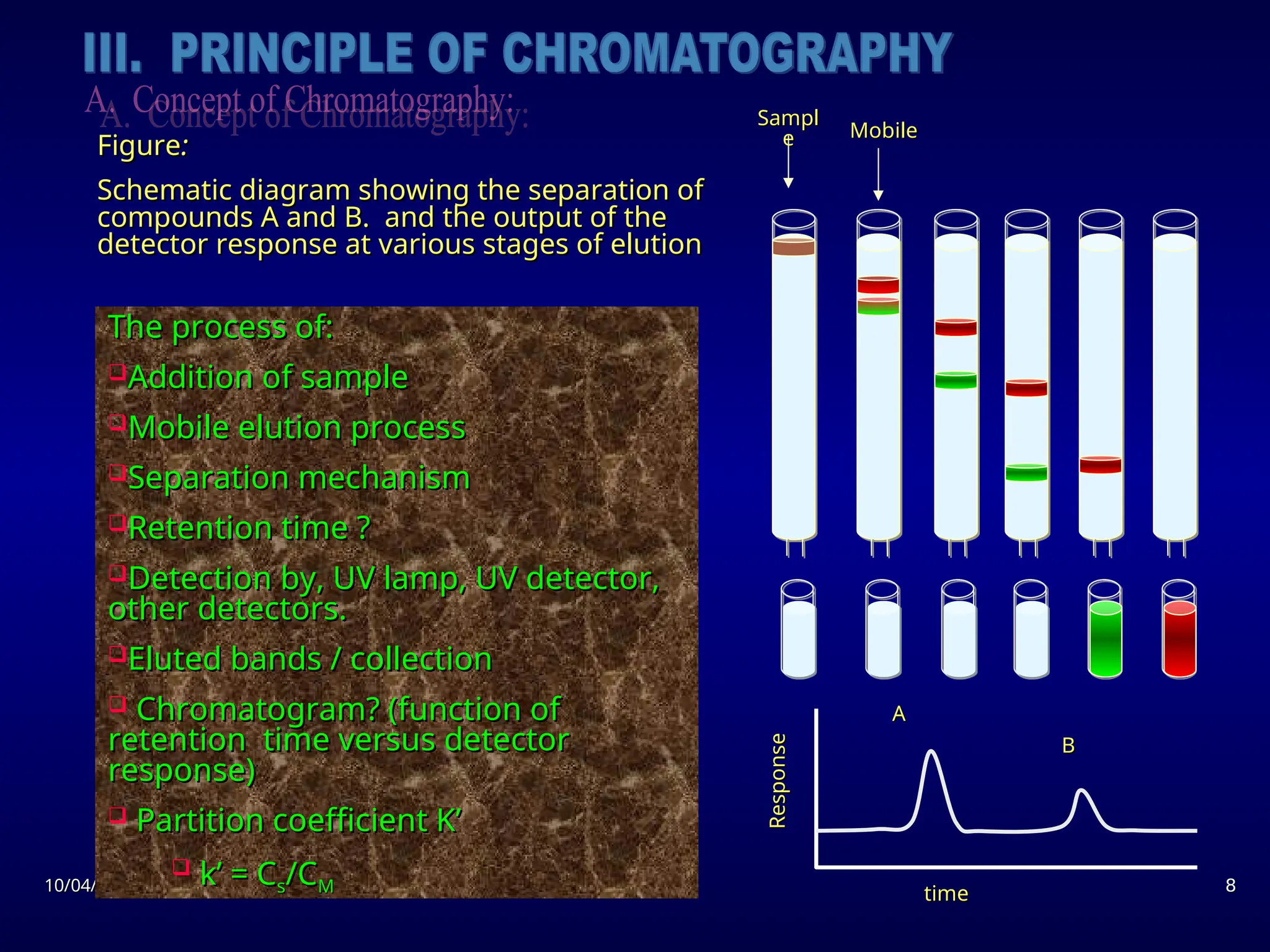
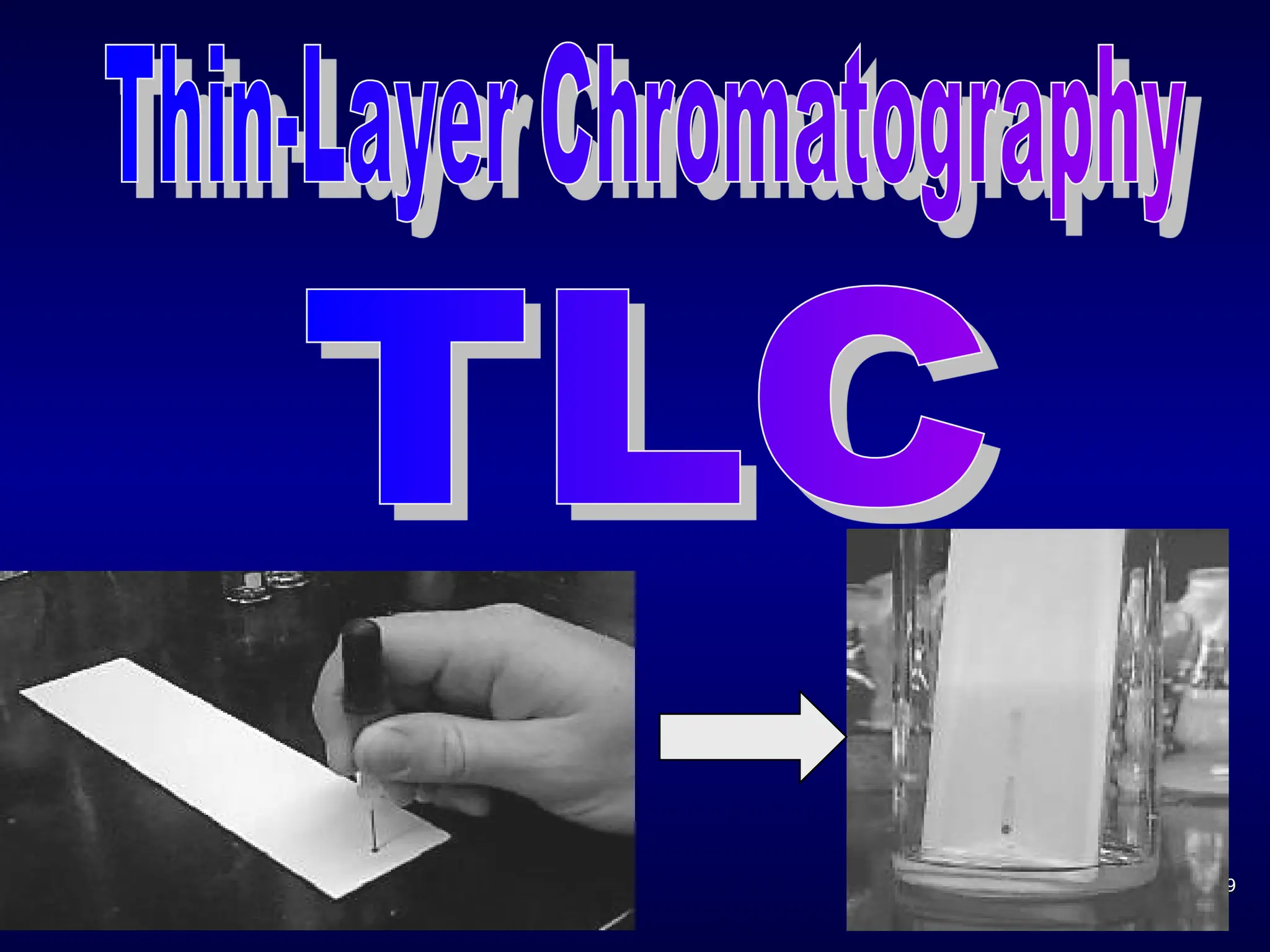
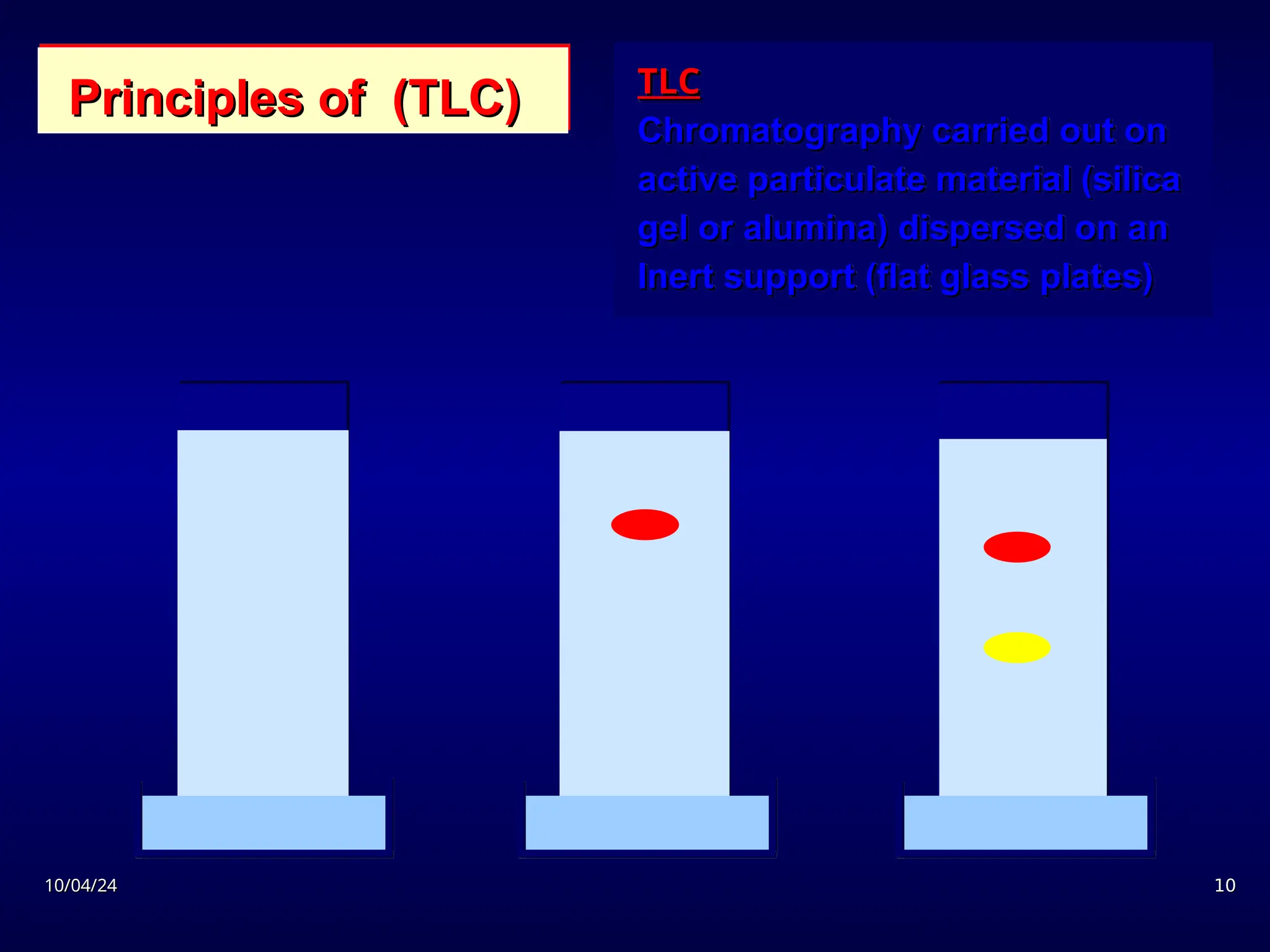
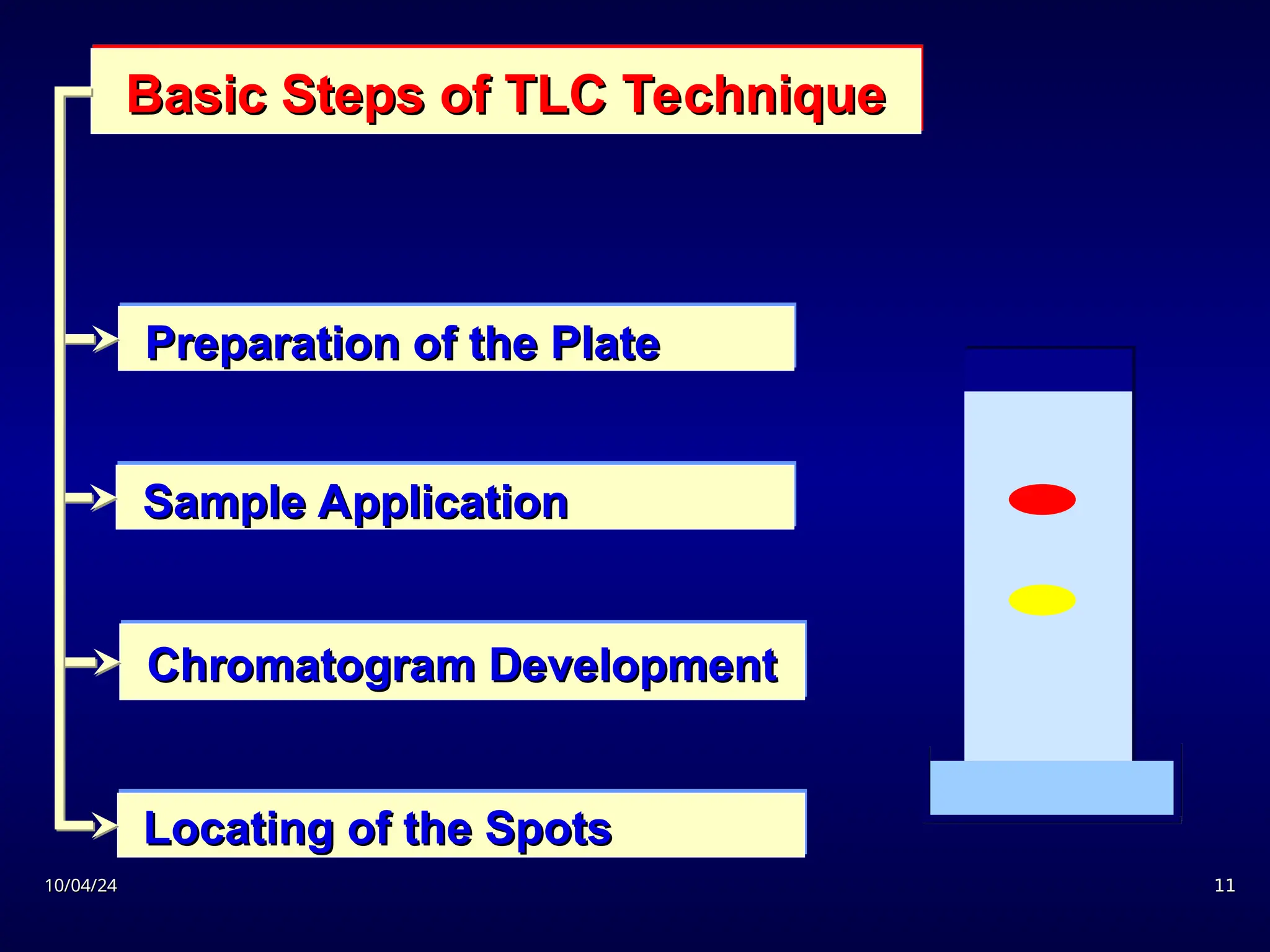
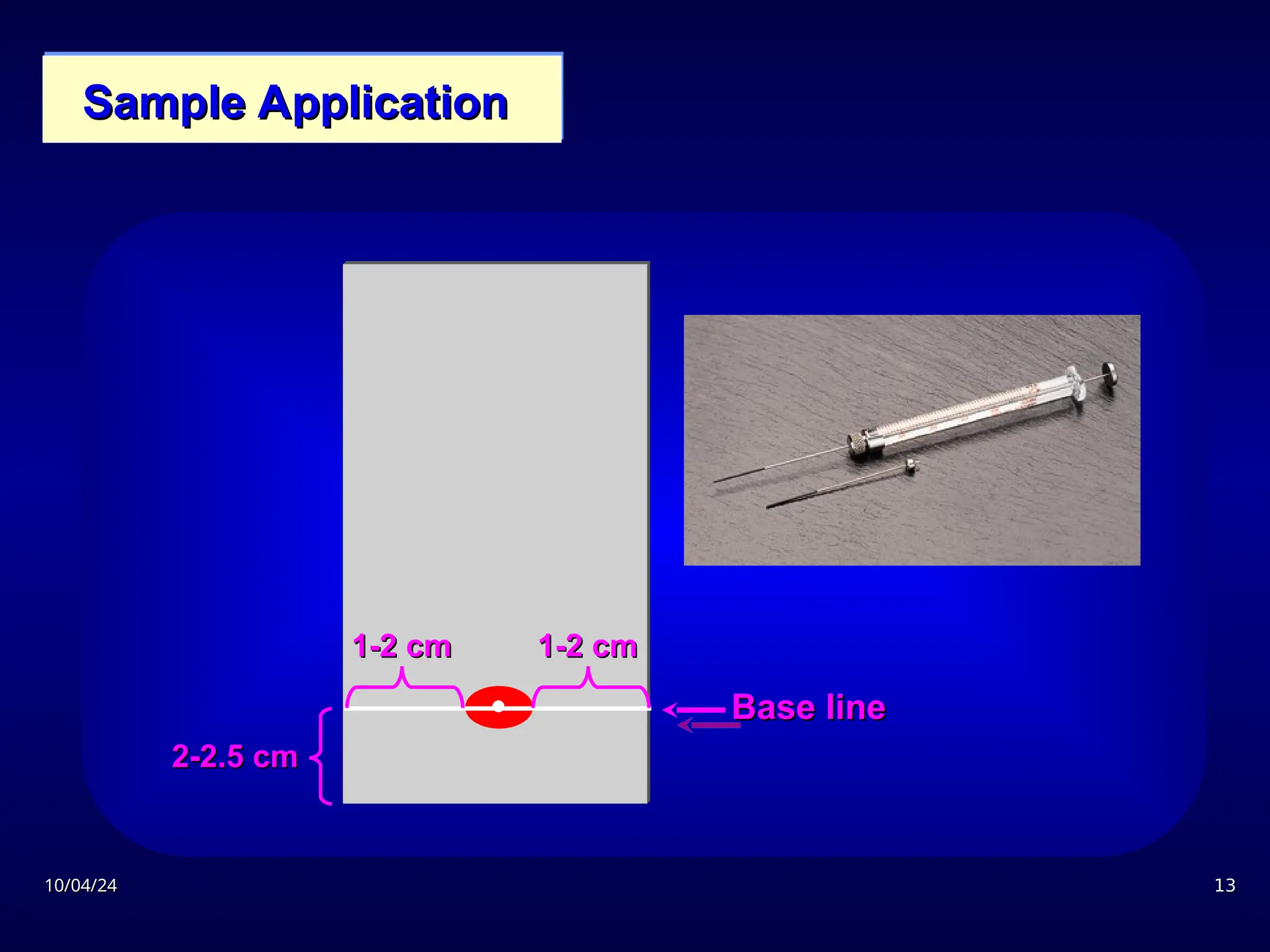
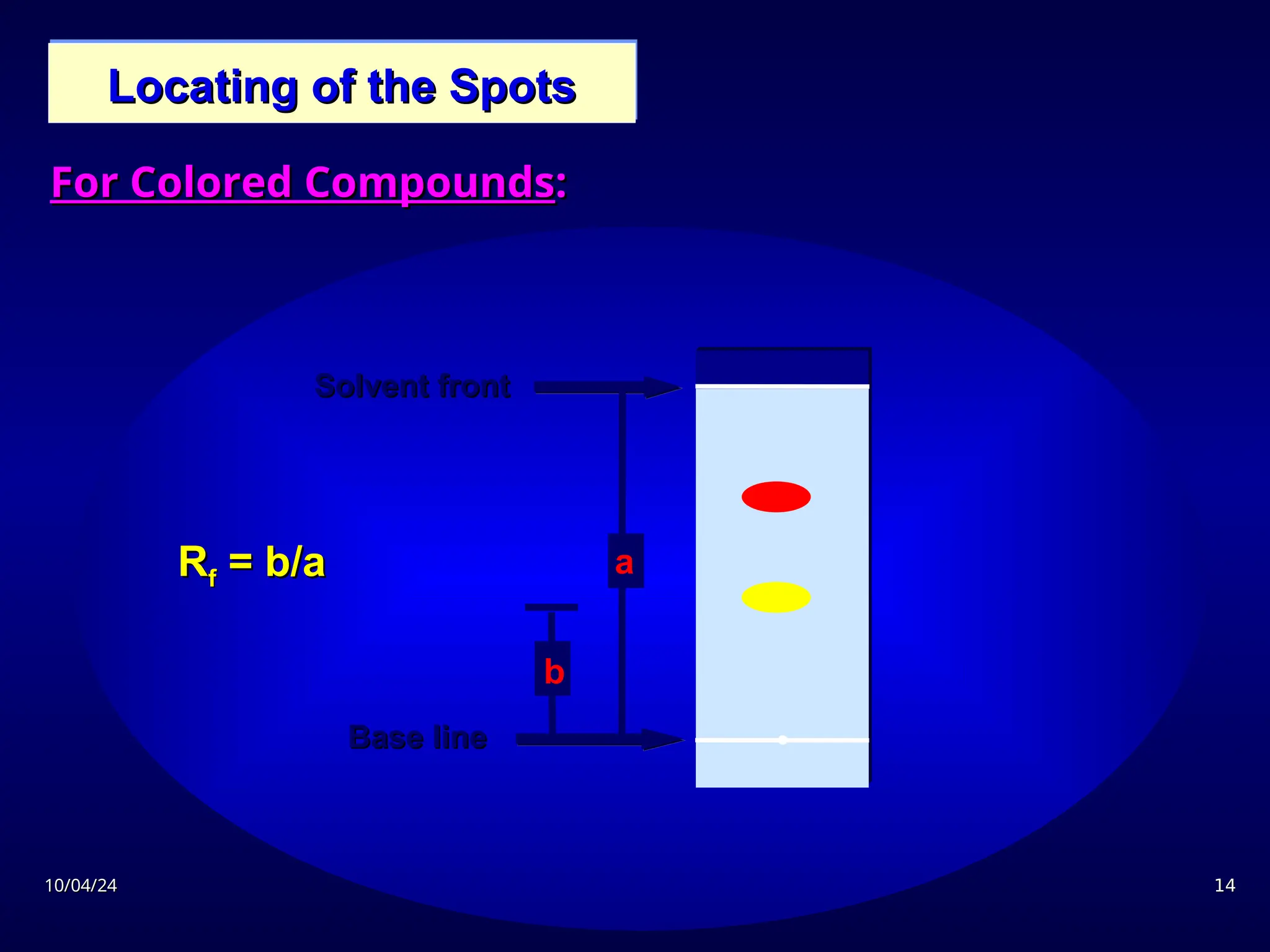
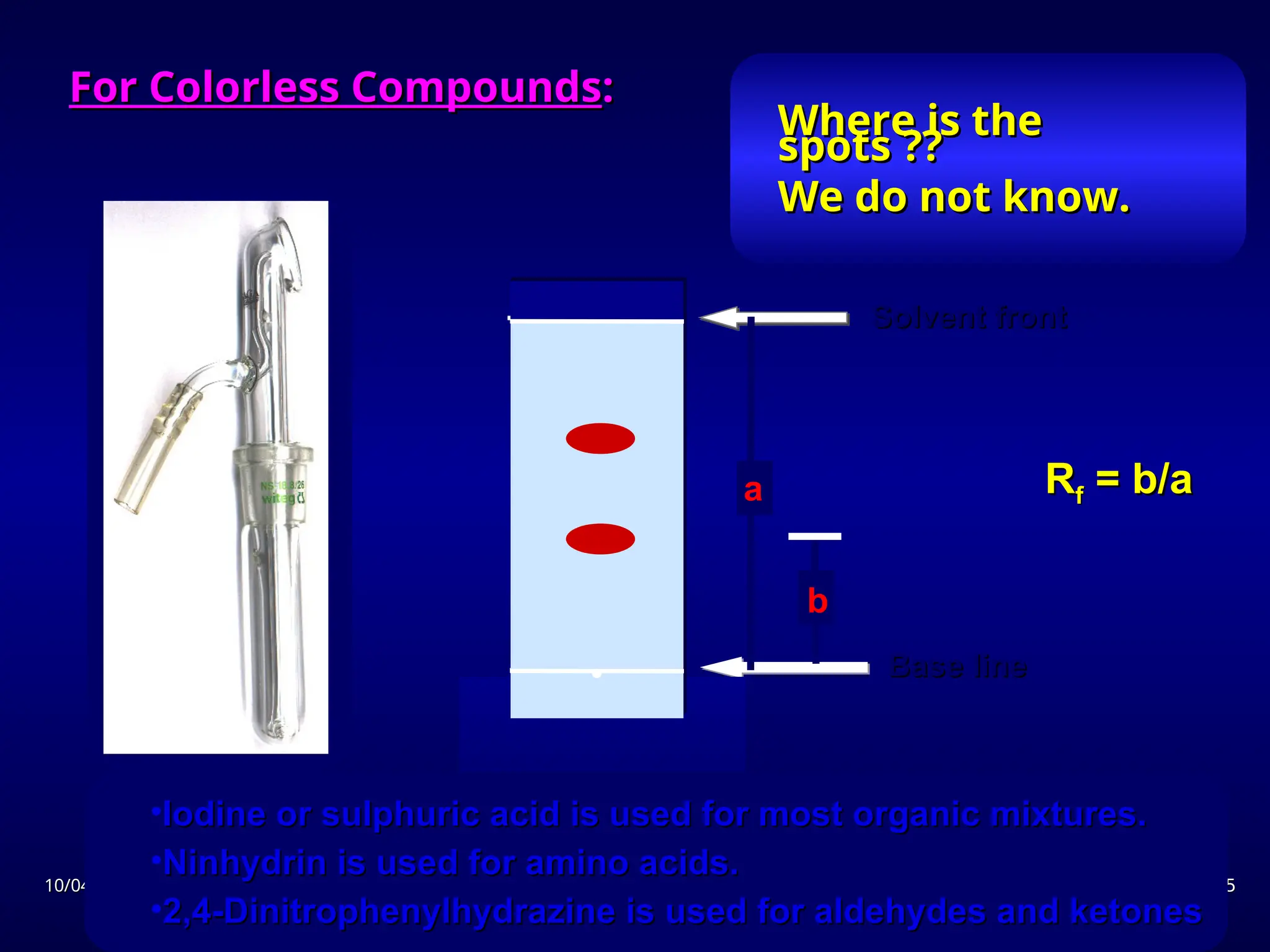
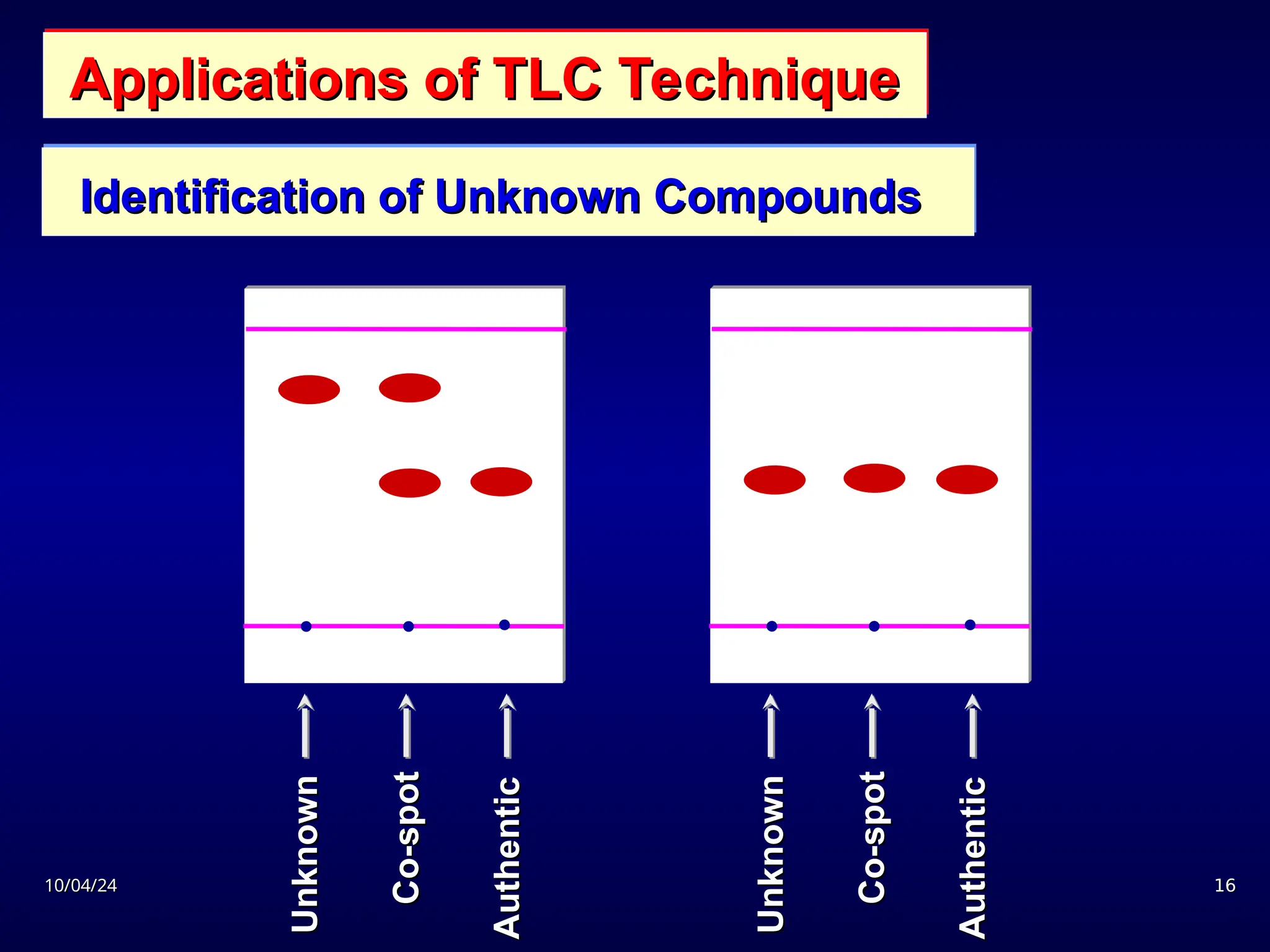
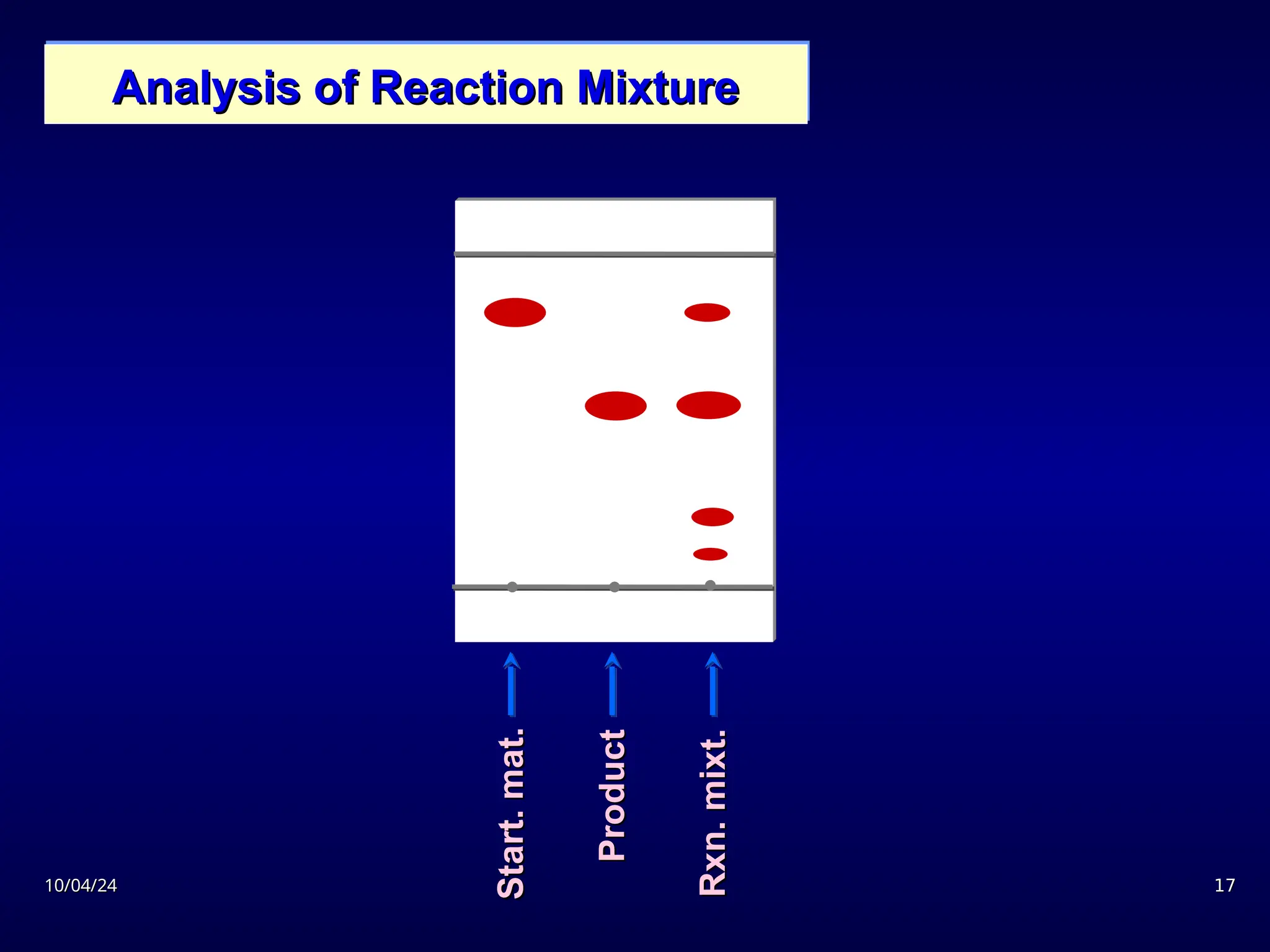
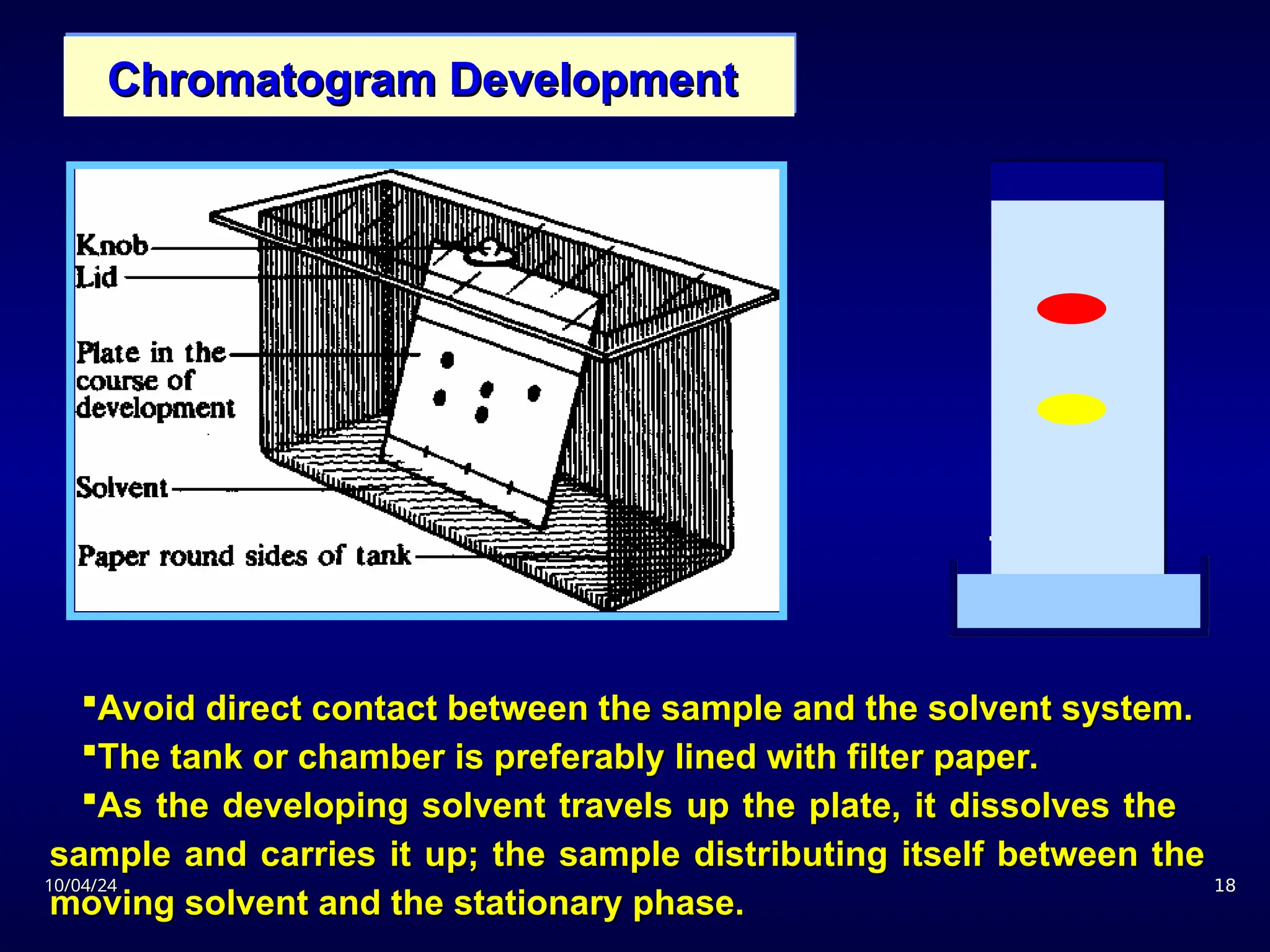
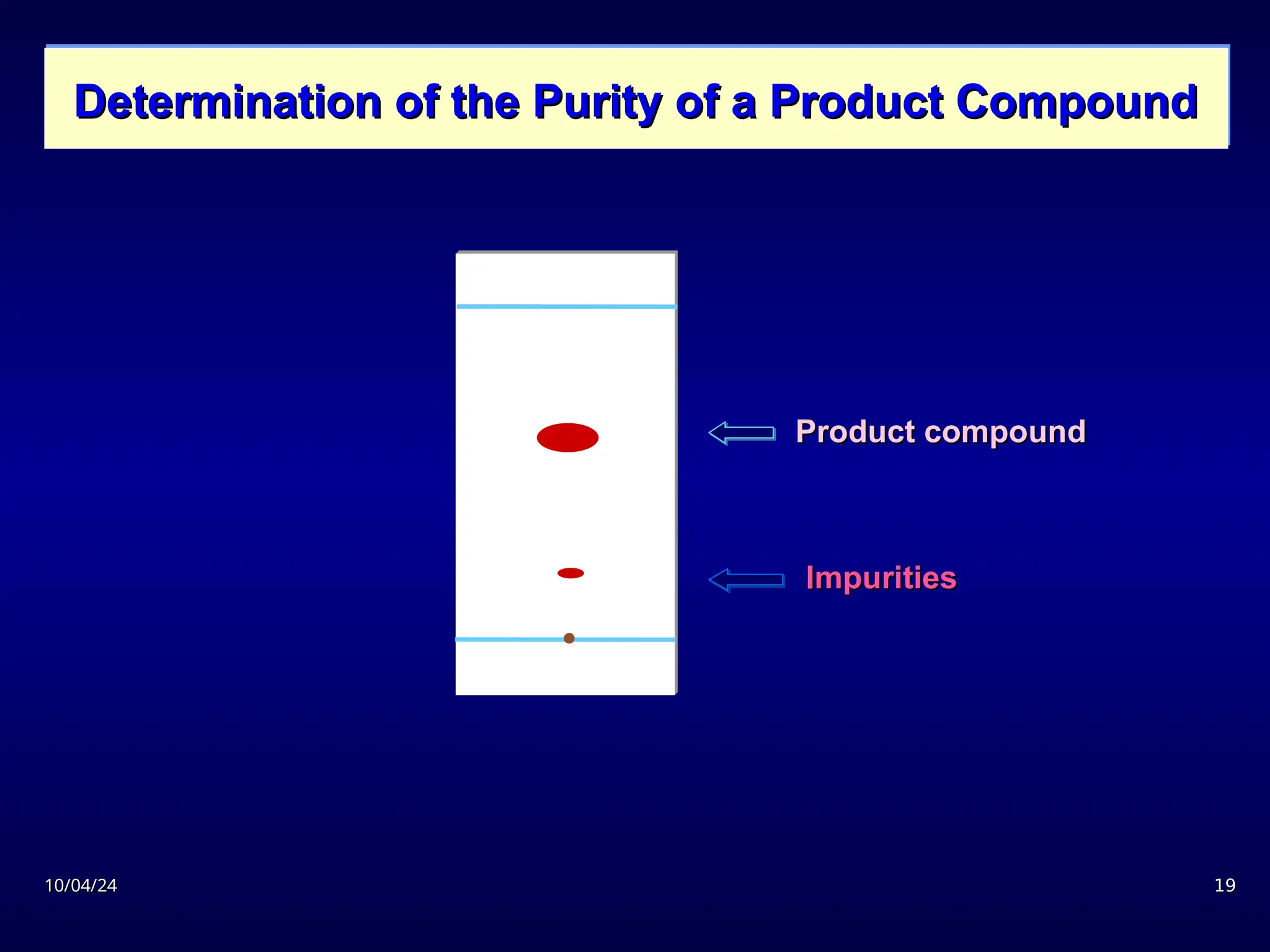
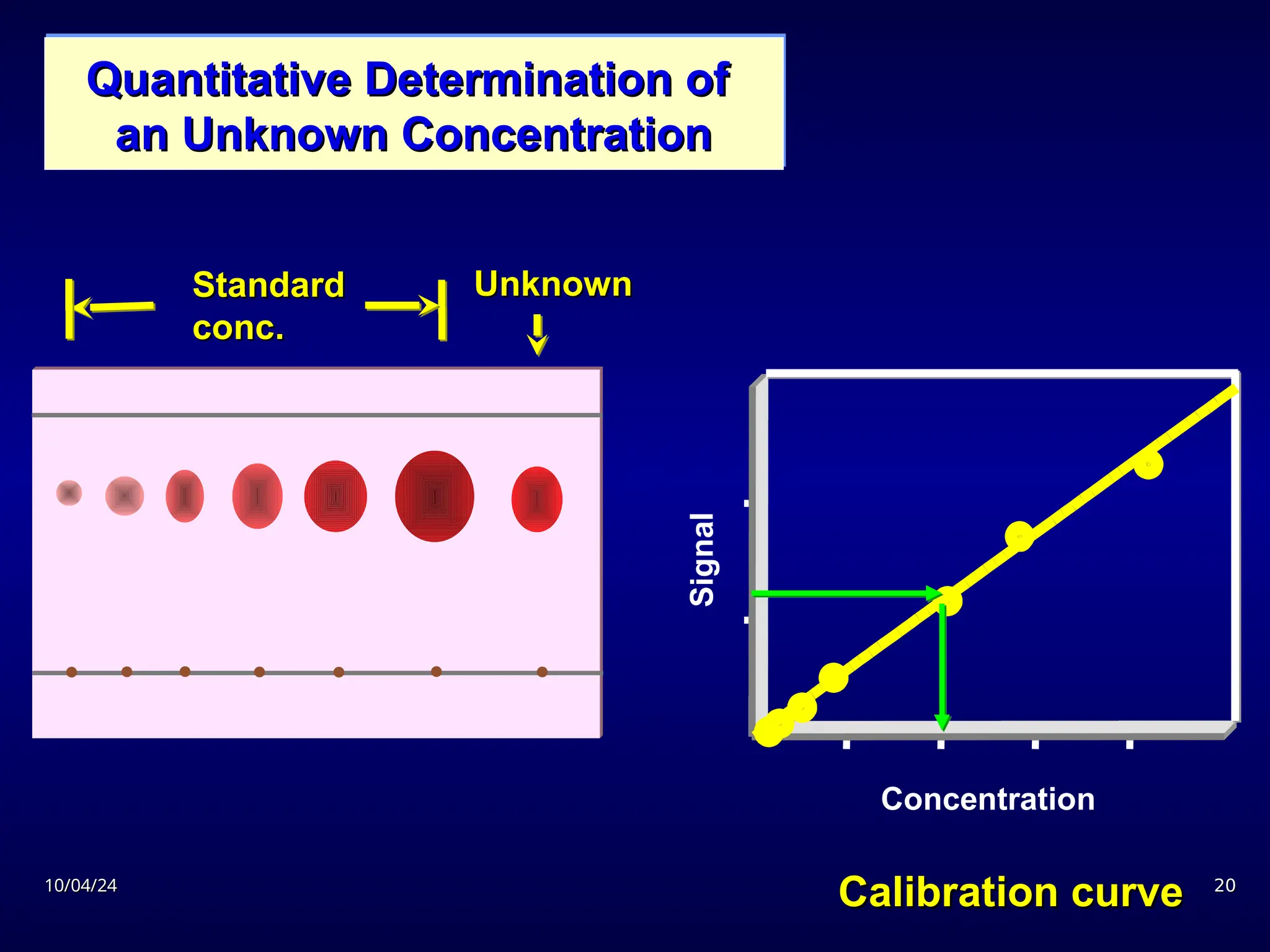
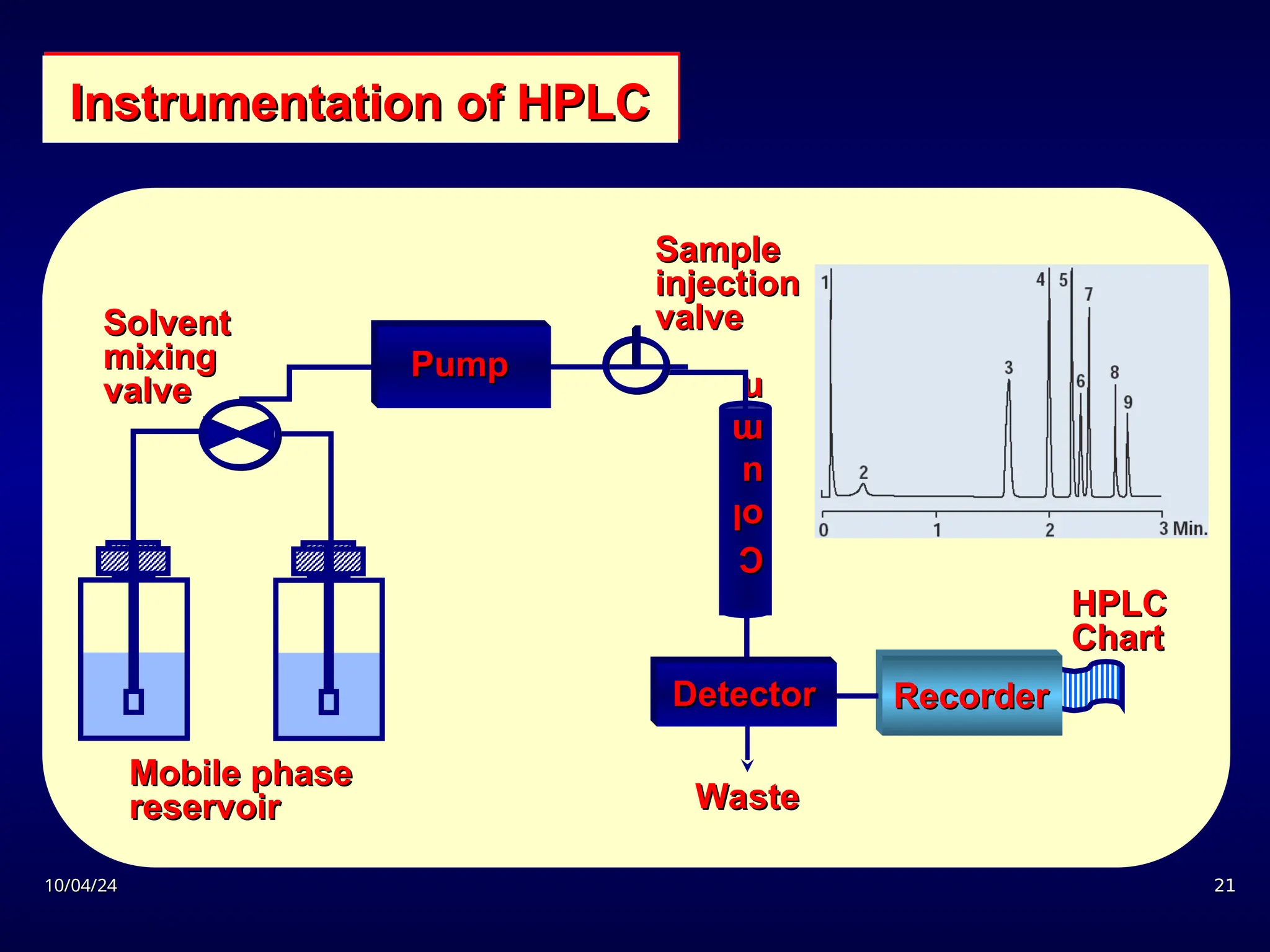
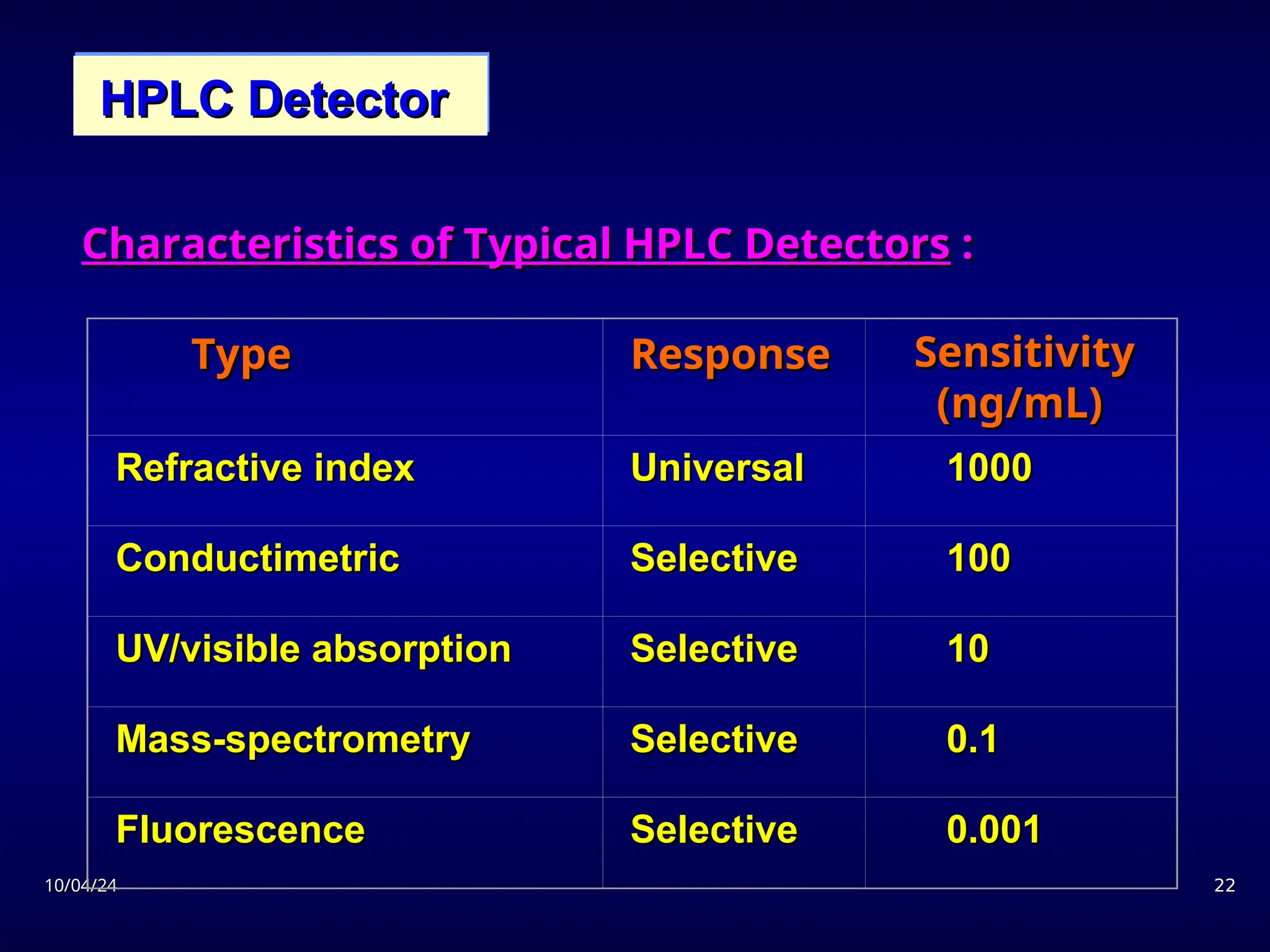
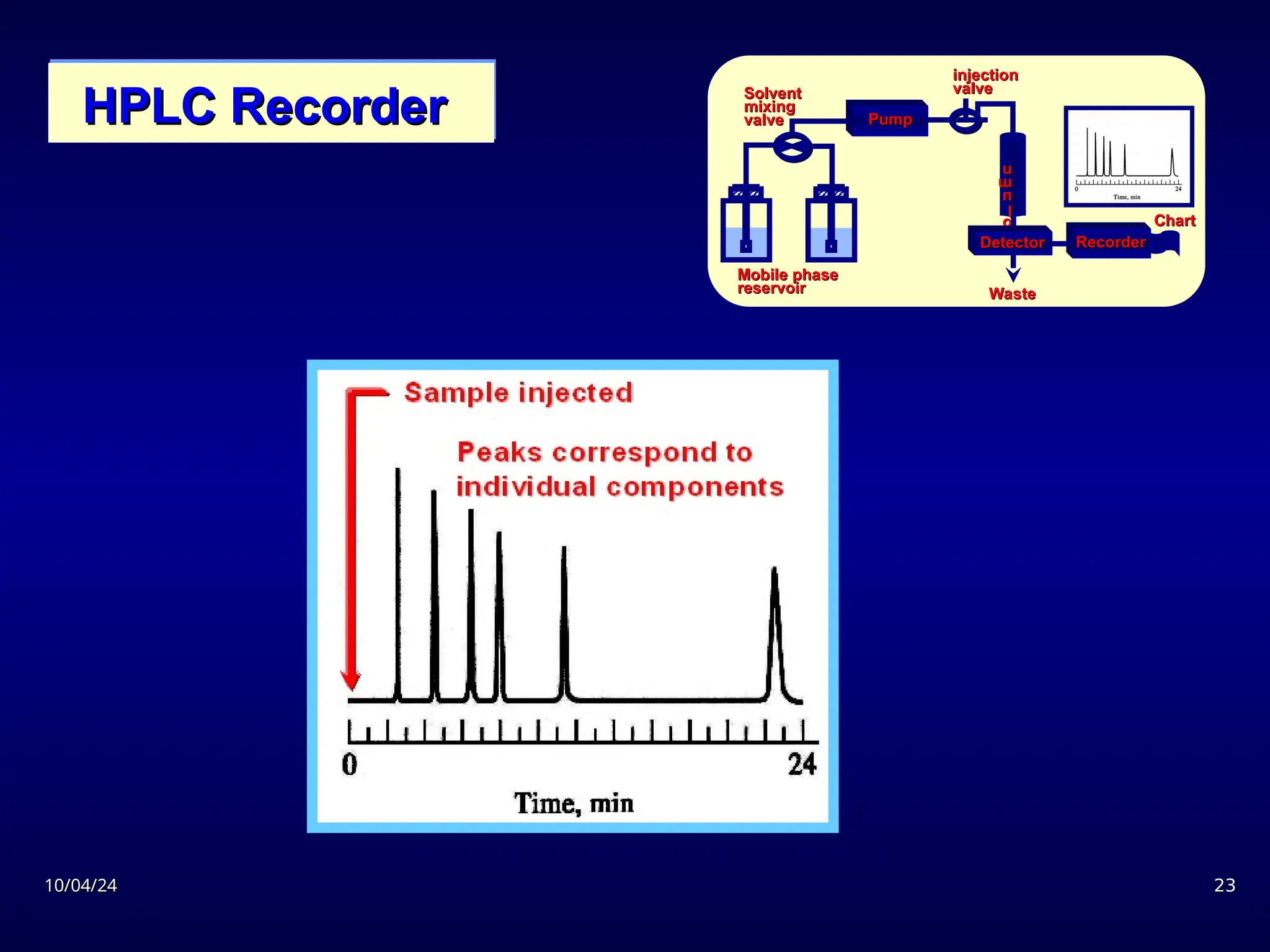
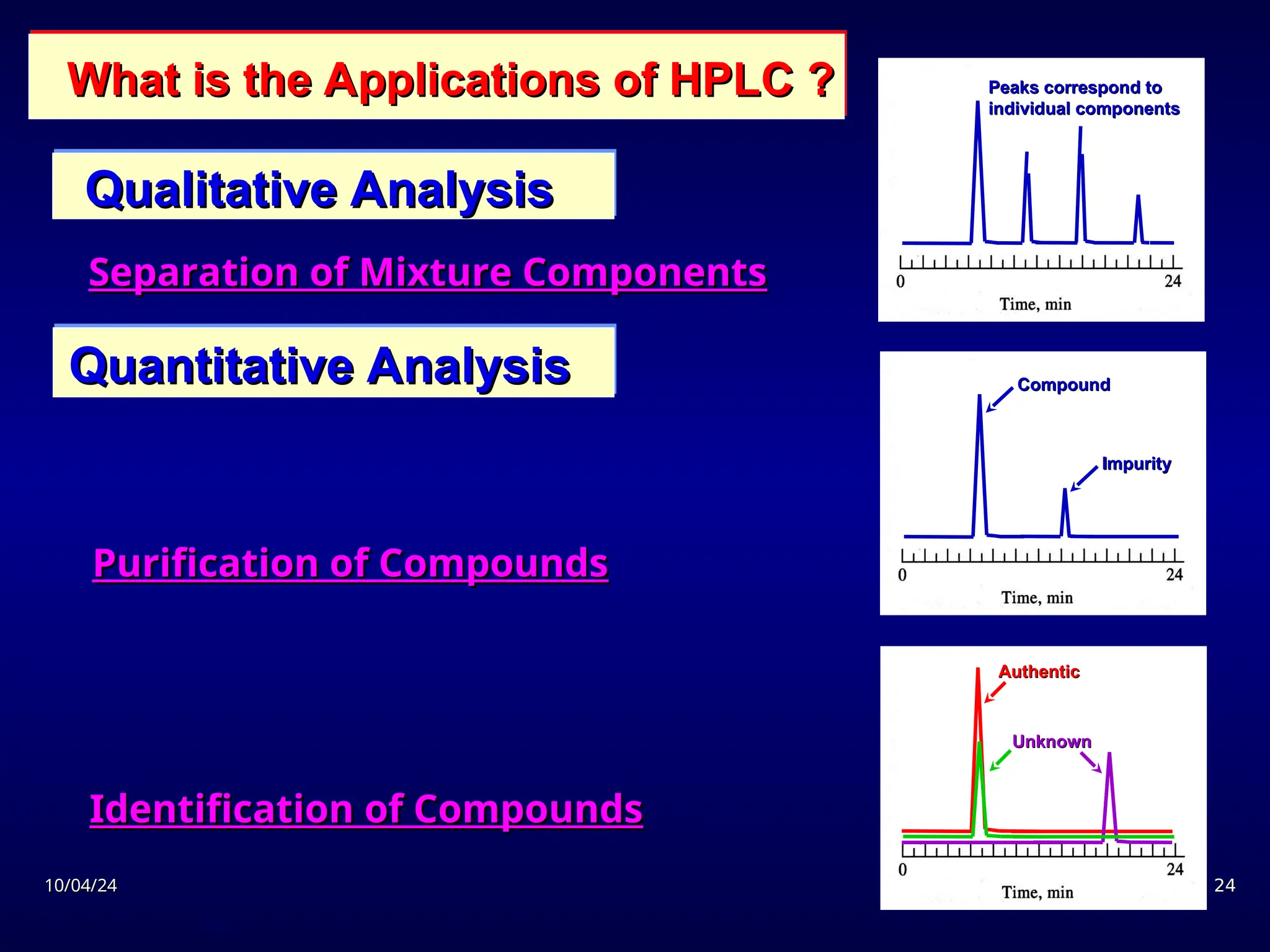
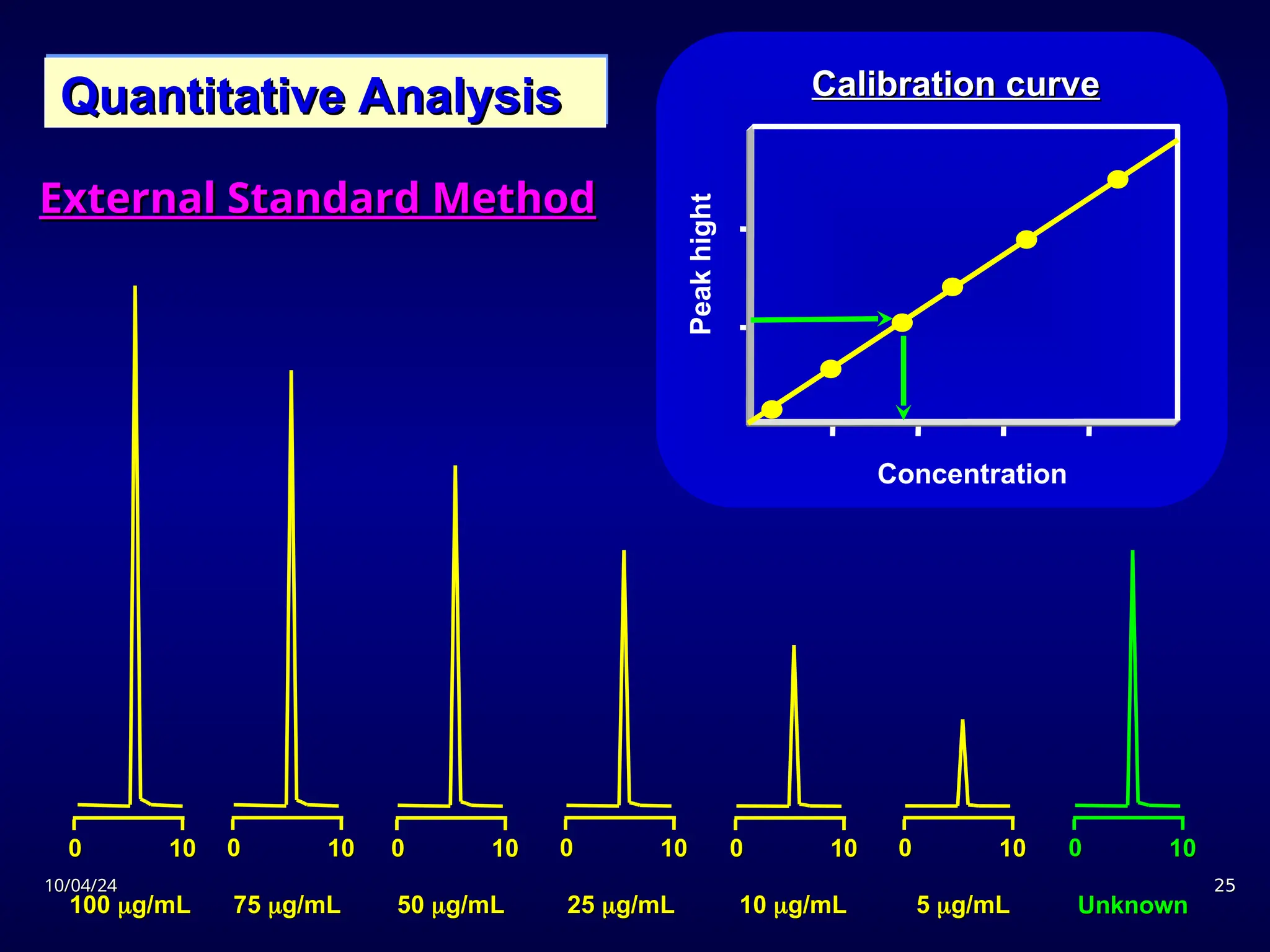
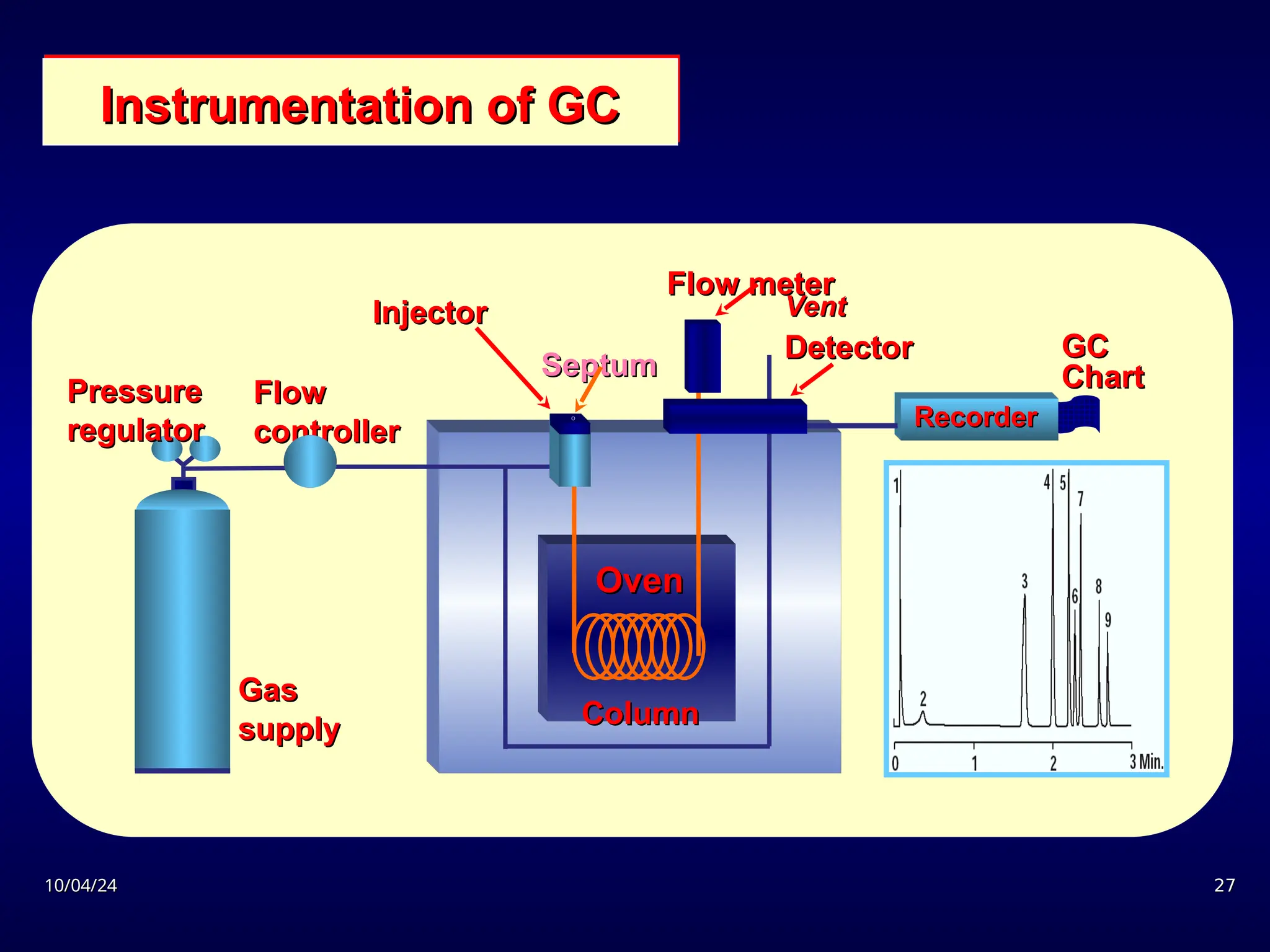
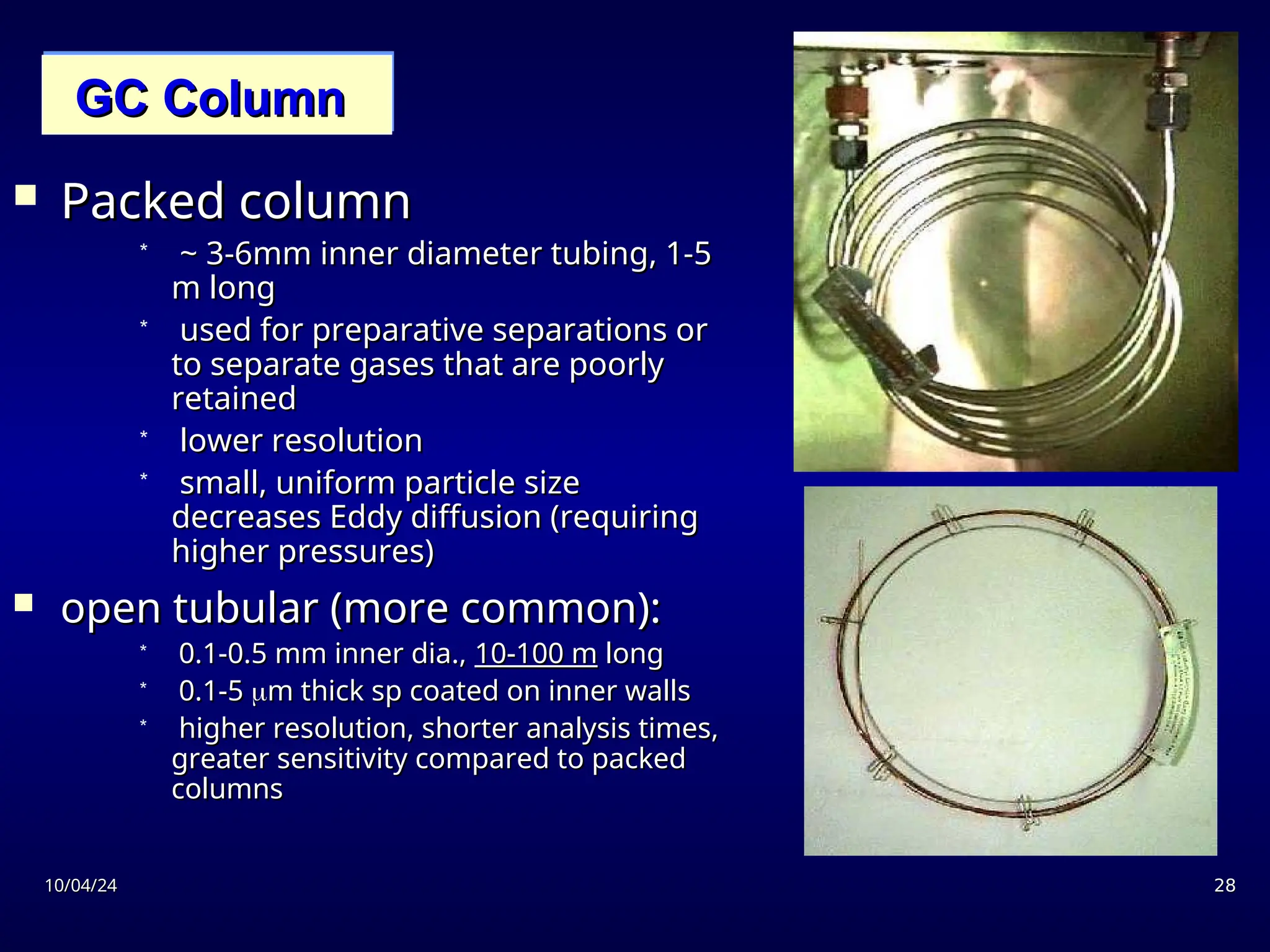
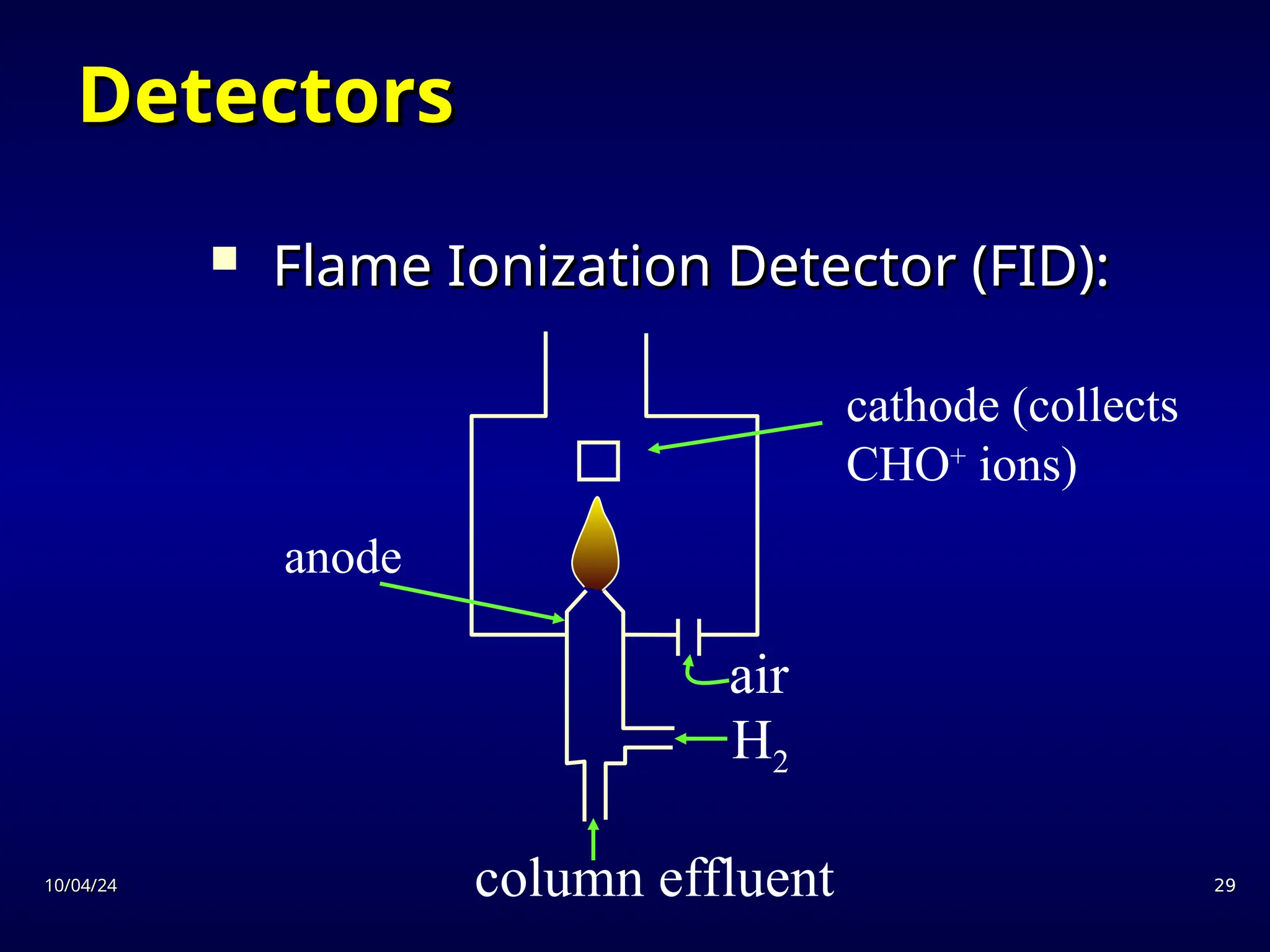
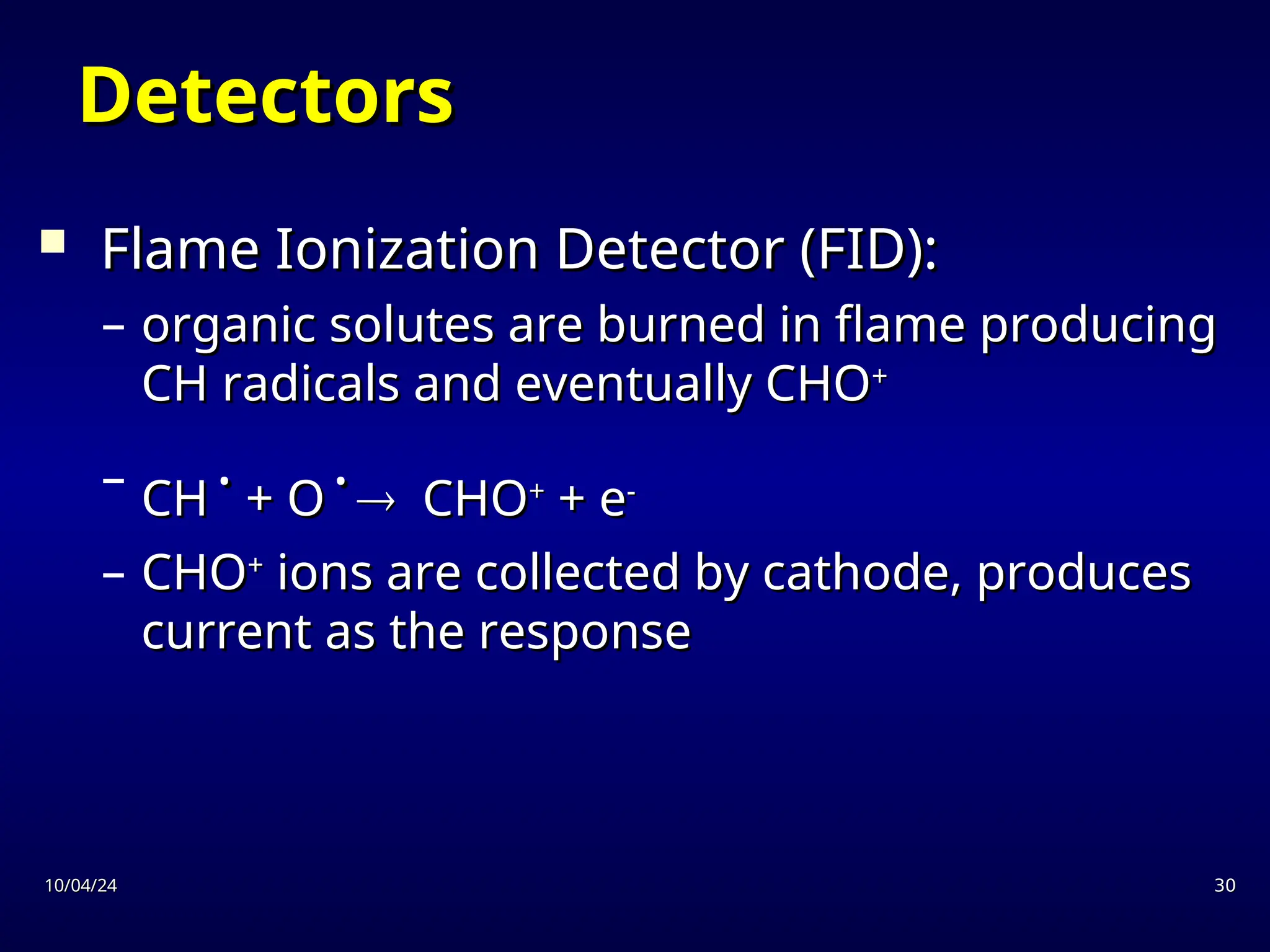
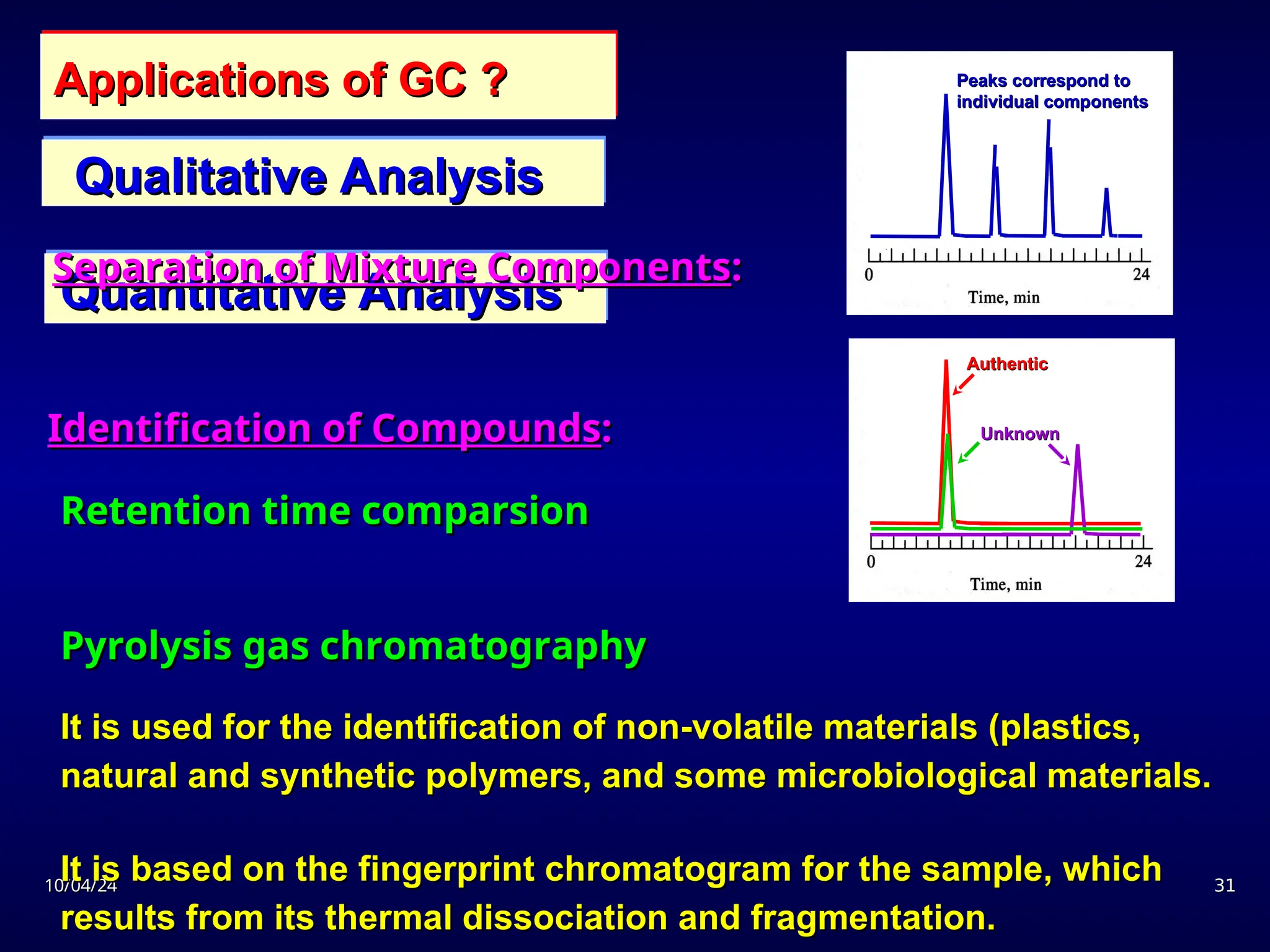
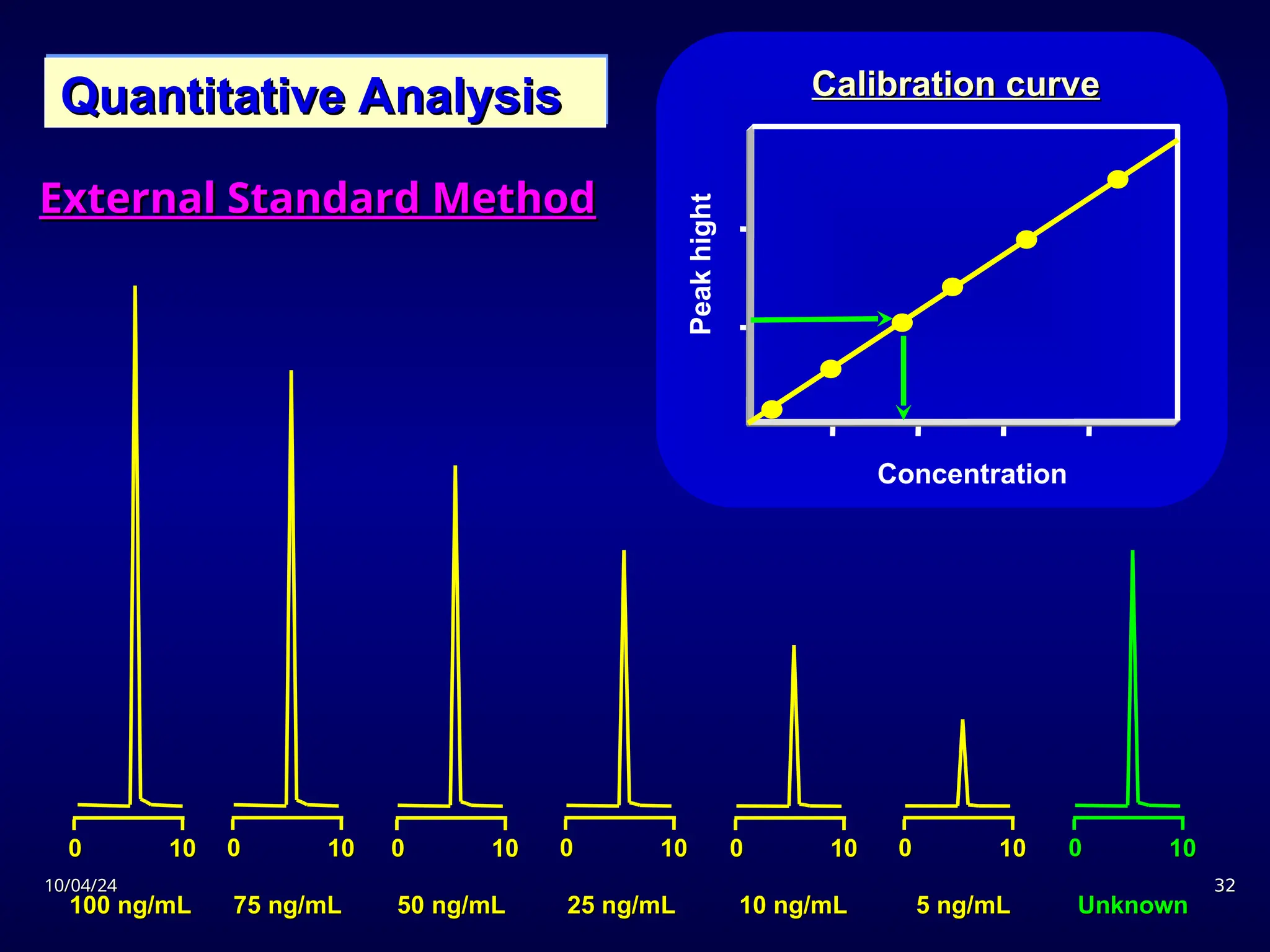
The document provides an overview of chromatography, detailing its definition, principles, and various techniques such as High Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), and Thin Layer Chromatography (TLC). It explains the roles of mobile and stationary phases, the separation mechanisms, and the applications of these methods in qualitative and quantitative analysis. Additionally, it discusses instrumentation and specific applications in drug, food, and environmental analyses.