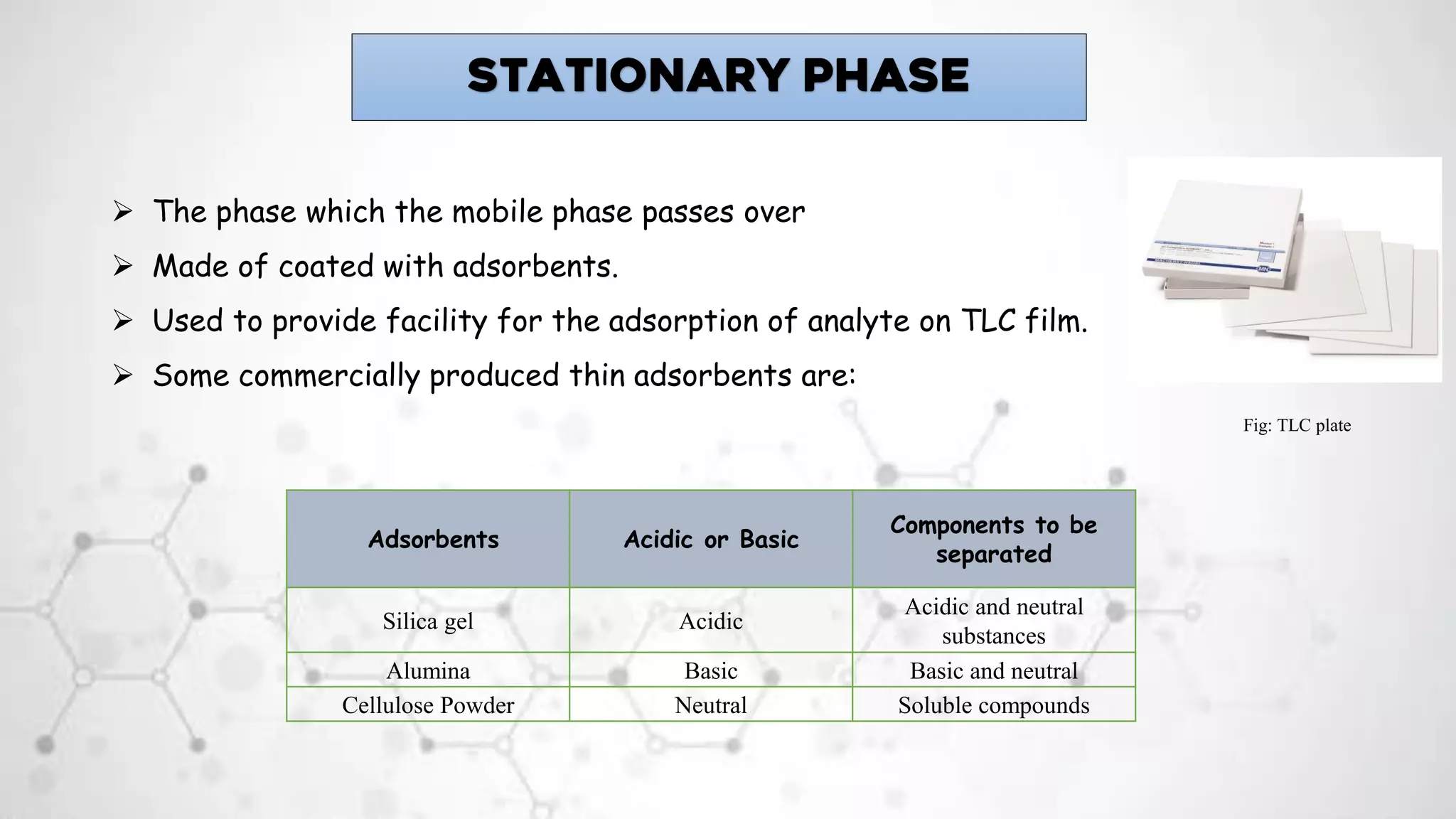
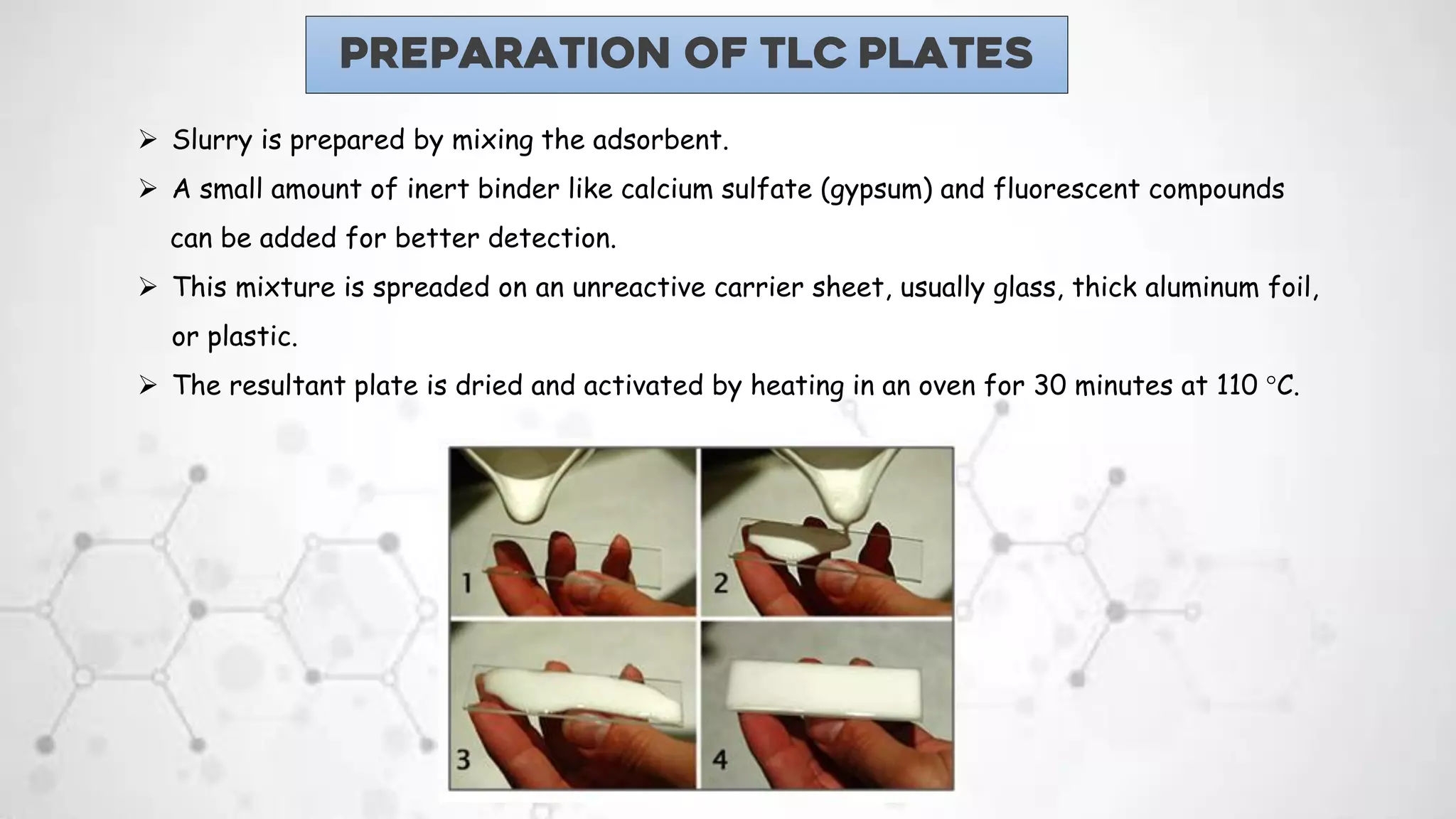
1. Thin layer chromatography uses a stationary phase, such as silica gel or alumina, coated onto a plate. A mobile phase, such as a solvent or solvent mixture, is run up the plate to separate the components of a sample spotted onto the plate.
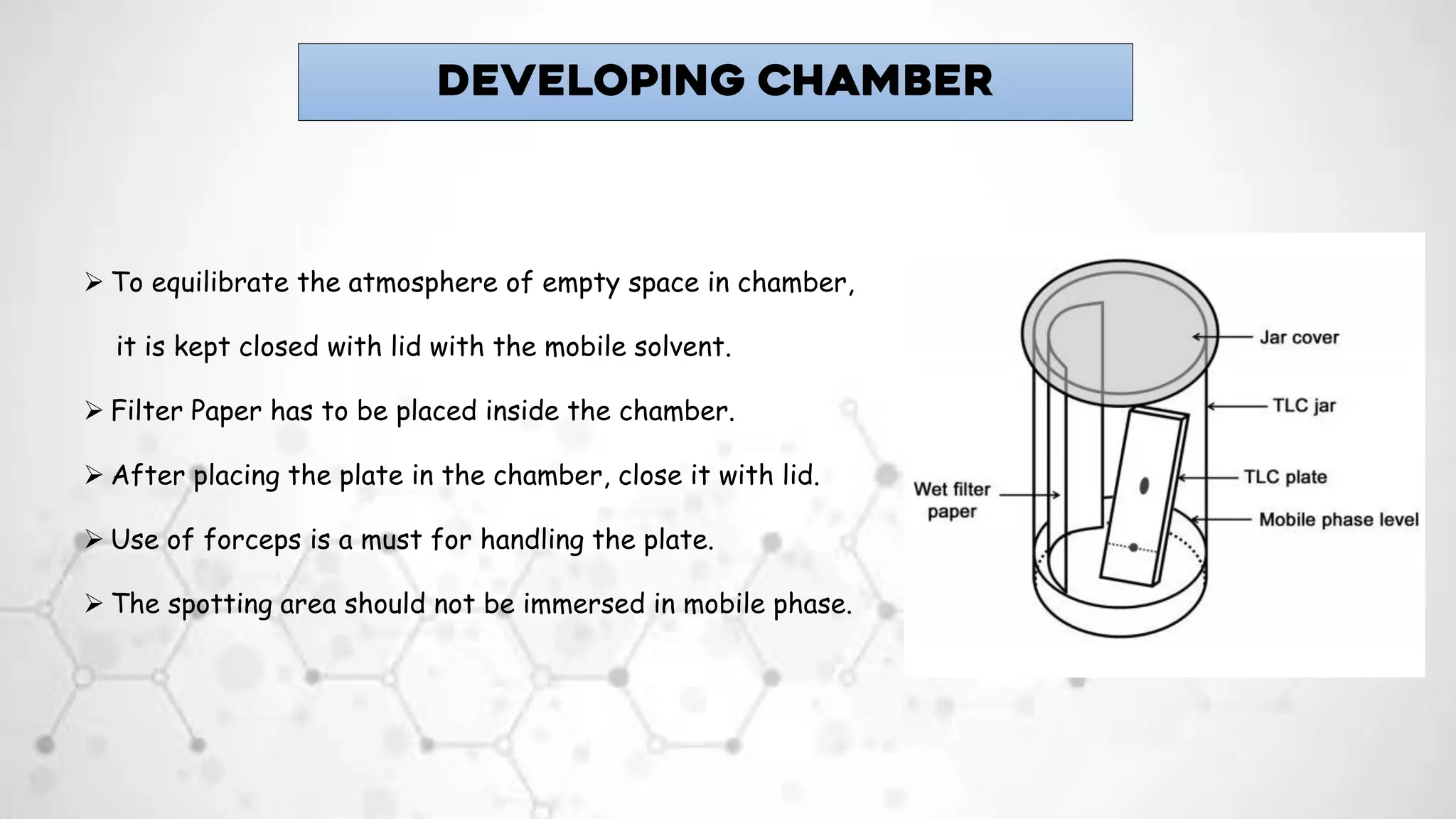
2. Key instruments used in TLC include TLC plates, forceps, pencil, ruler, filter paper, micropipette, and a developing chamber. Samples are spotted onto the plate using a micropipette and developed in the chamber containing the mobile phase and filter paper.
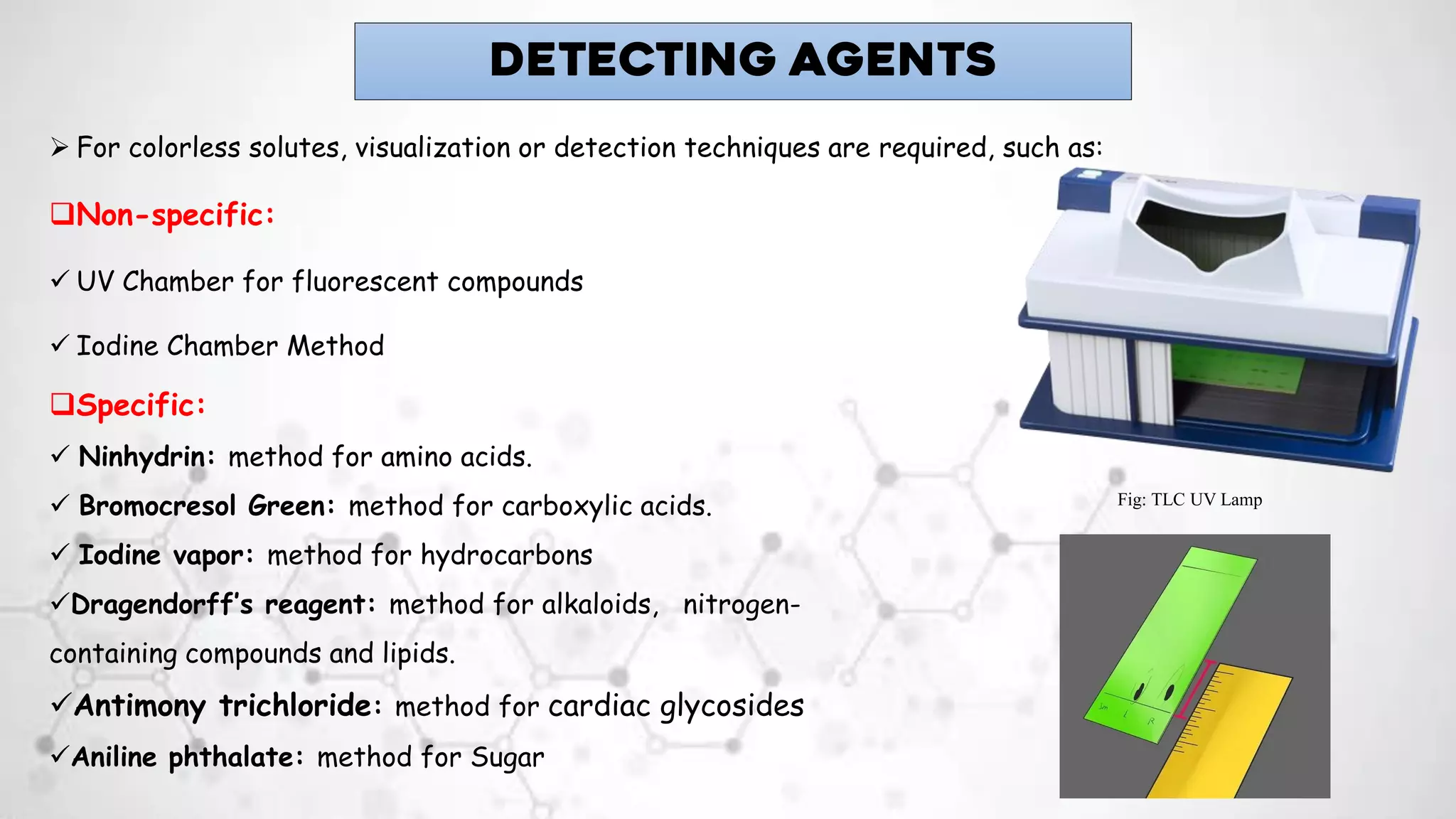
3. Detection of separated components is done using visualizing agents like iodine or UV light, or specific reagents that react with certain functional groups to