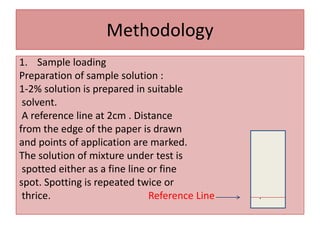
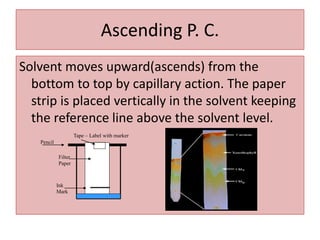
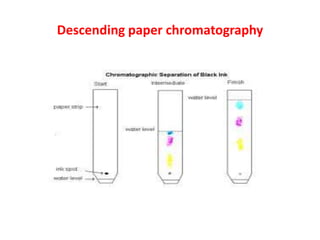
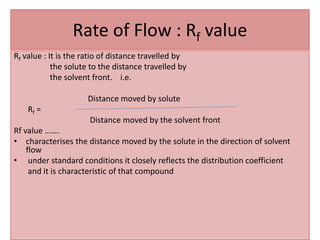
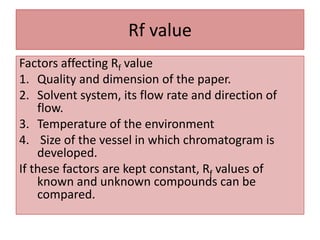
Paper chromatography is a planar chromatography technique using paper as a stationary phase to separate compounds based on their partitioning between stationary and mobile phases. It involves methodologies such as sample loading, solvent development, and spraying, with various solvent systems utilized to enhance separation. While it offers advantages of simplicity and cost-effectiveness, it has limitations like time consumption and inability to use certain detection methods.