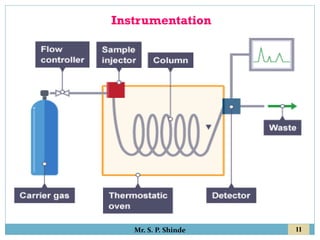
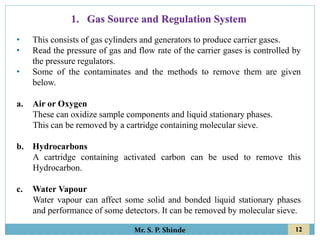
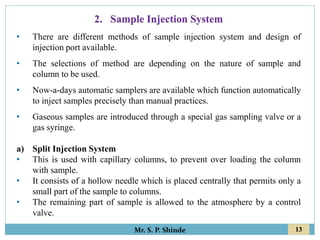
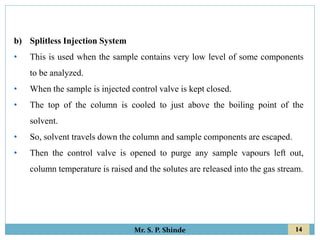
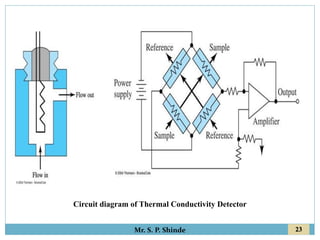
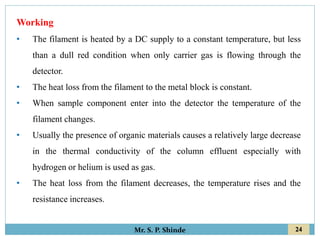
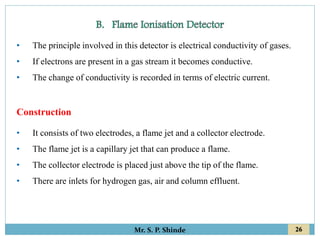
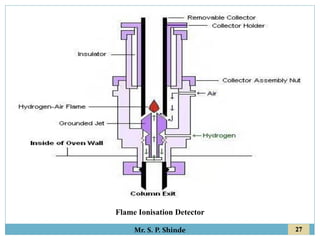
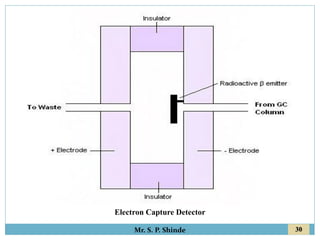
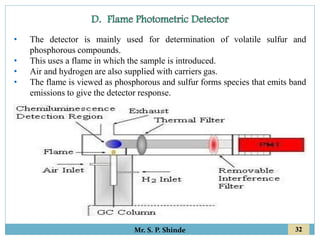
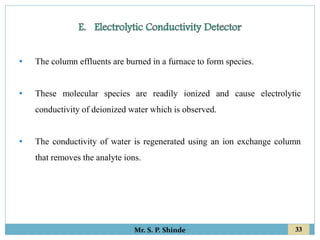
This document provides information about gas chromatography. It discusses the key components of a gas chromatography system including the mobile phase, stationary phase, columns, temperature control, sample injection systems, and various detectors. The mobile phase is usually an inert gas like helium, hydrogen, or nitrogen. Stationary phases can be solid adsorbents or liquid coatings. Columns include packed columns and capillary columns. Temperature control programs are used to separate compounds of varying boiling points. Common detectors mentioned are the thermal conductivity detector, flame ionization detector, and electron capture detector.