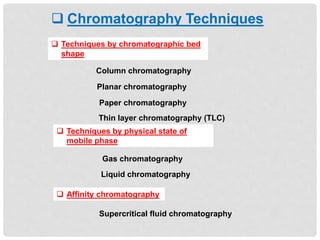
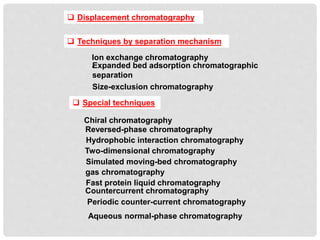
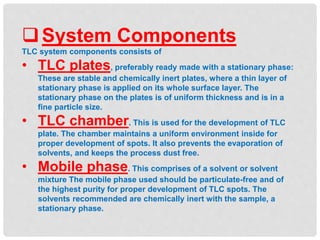
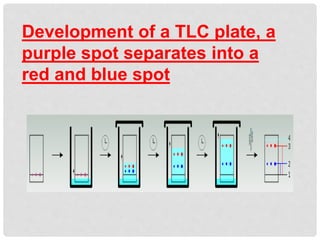
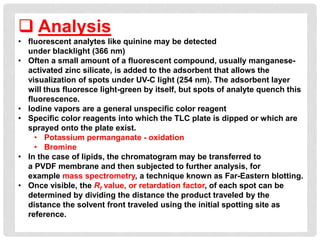
Chromatography is a laboratory technique used for separating mixtures based on the differential movement of compounds through a mobile phase and a stationary phase. Thin Layer Chromatography (TLC), a type of planar chromatography, is widely used for analyzing mixtures and is characterized by its quick development time, simplicity, and efficiency in compound visualization and identification. It employs various techniques, with applications in purity checking, compound identification, and sample purification.