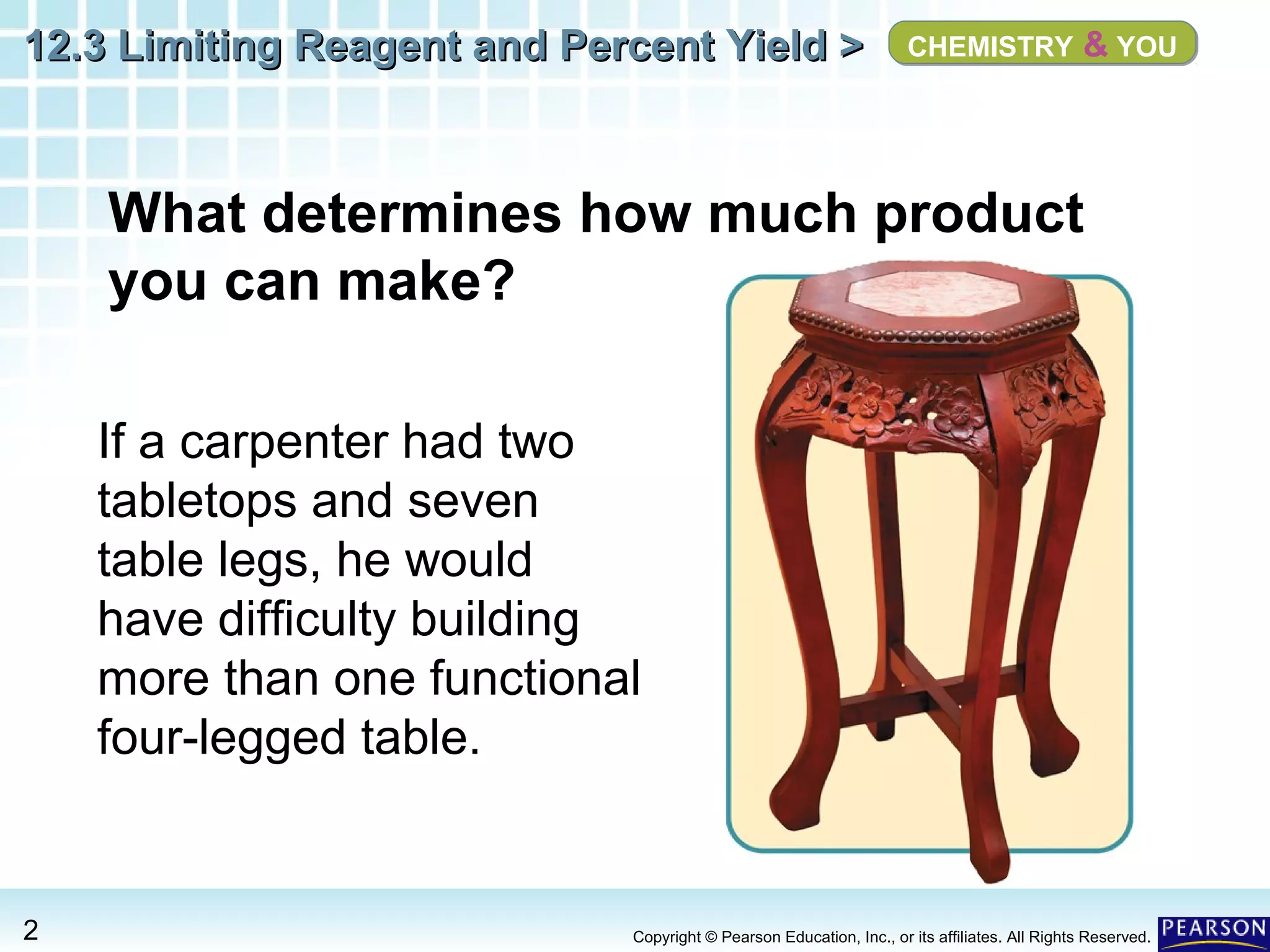
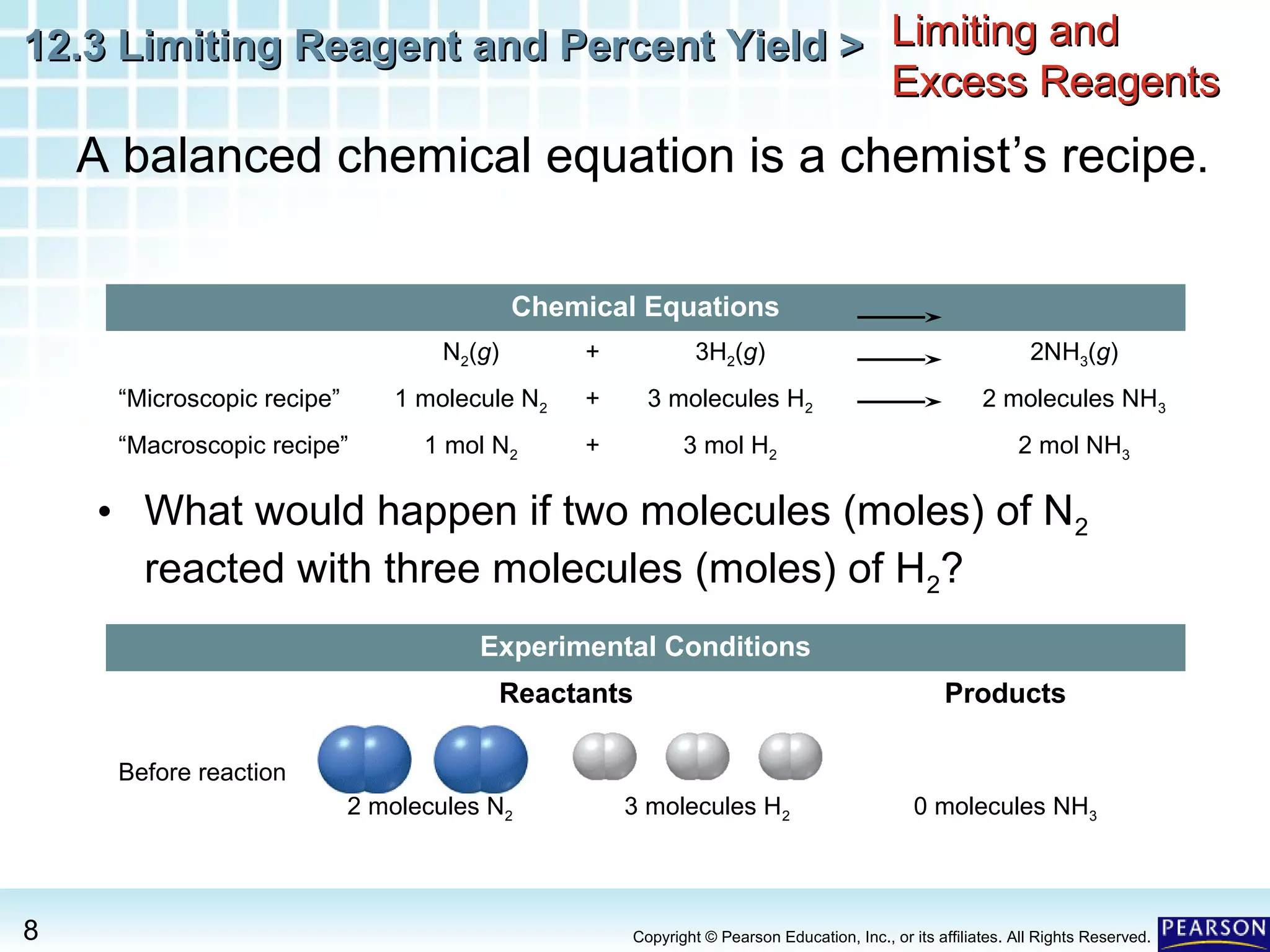
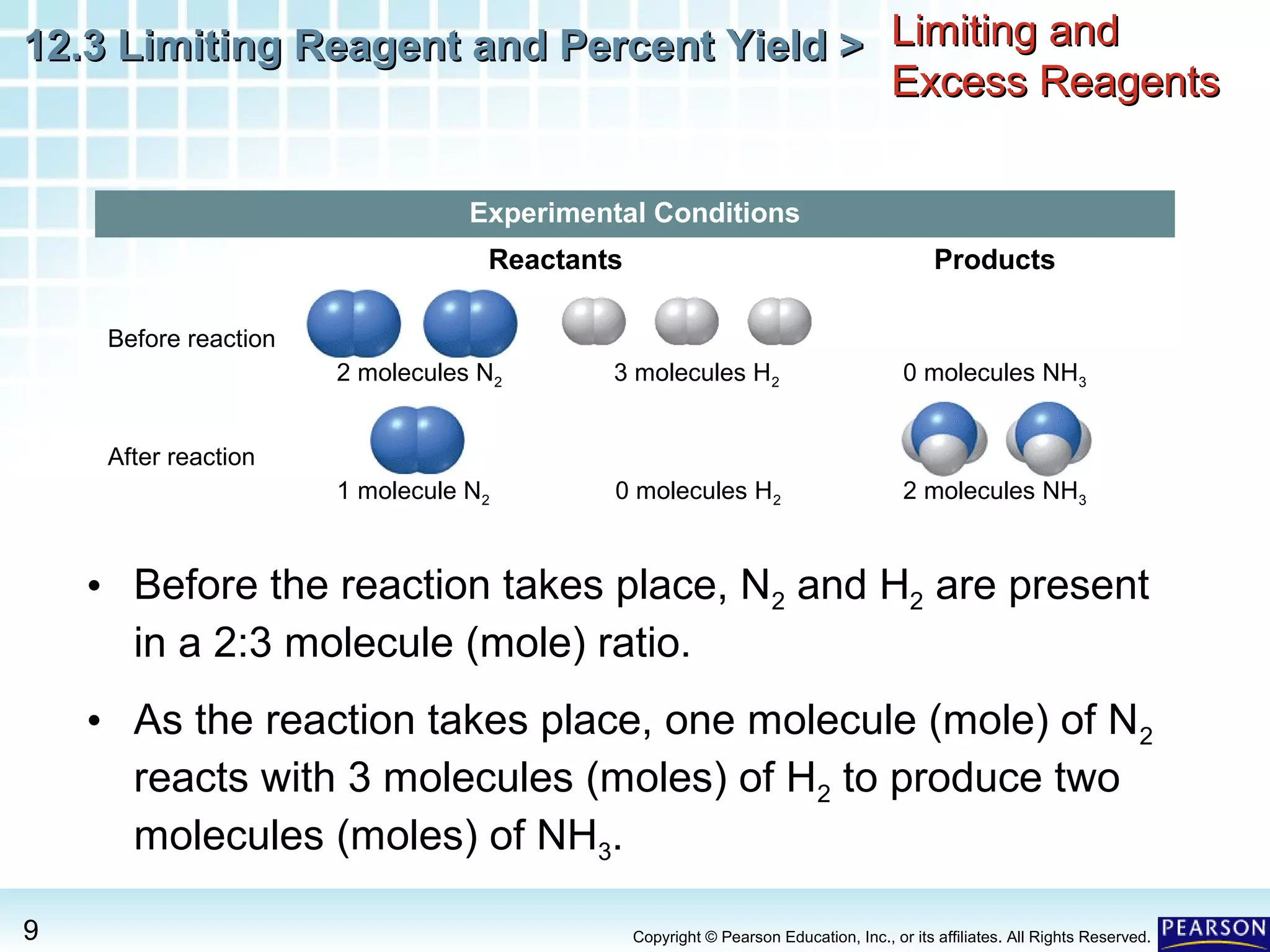
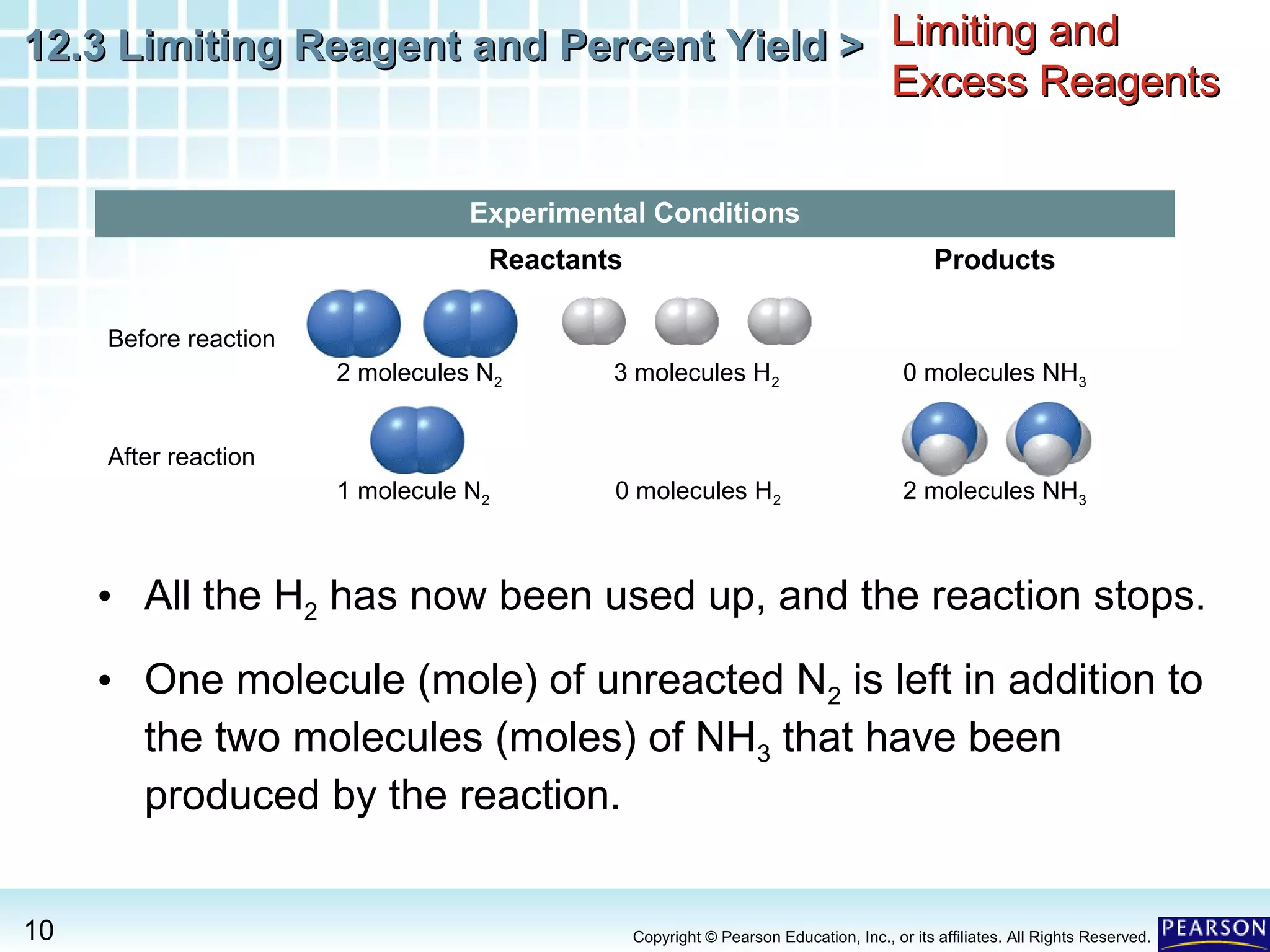
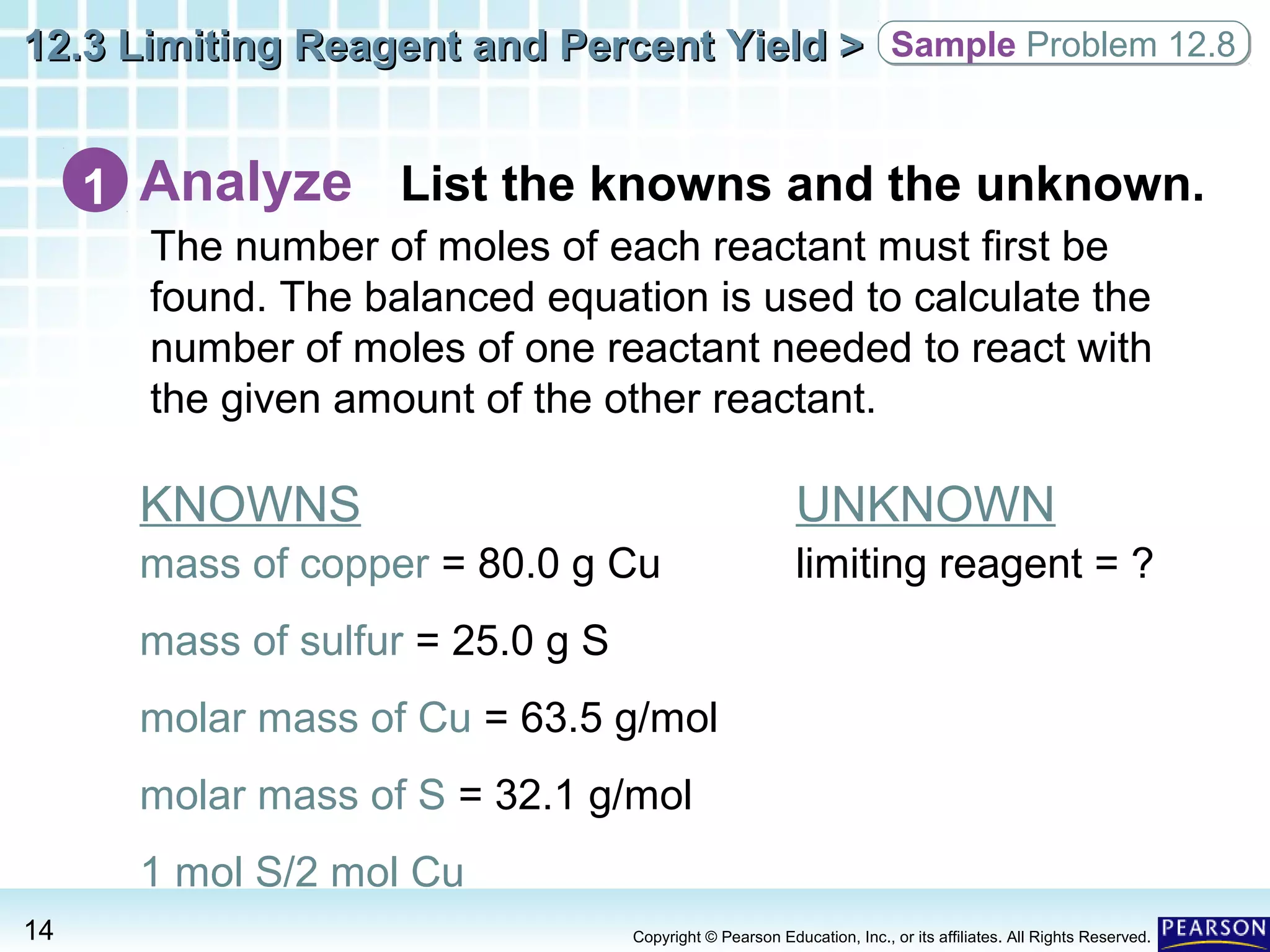
The document discusses limiting reagents, excess reagents, theoretical yield, actual yield, and percent yield in chemical reactions. It explains that the limiting reagent determines how much product can be formed. The excess reagent is not completely used up. Percent yield compares the actual yield to the theoretical yield and measures the reaction's efficiency.